- Schizophrenia
2018-11-07 11:07:34
Today's Topics
Schizophrenia
Simulating the Experience
Overview
- Lifetime prevalence ~ 0.3-0.7%
- Broader definitions suggest 2-3 or 3-5%
- ~1/3 chronic & severe
- Onset post-puberty, early adulthood
- Males earlier onset & greater severity
- Pervasive disturbance in mood, thinking, movement, action, memory, perception
- Increased (early) mortality
"Positive" symptoms
- “Additions” to behavior
- Disordered thought
- Delusions of grandeur, persecution
- Hallucinations (usually auditory)
- Bizarre behavior
"Negative" symptoms
- “Reductions” in behavior
- Poverty of speech
- Flat affect
- Social withdrawal
- Impaired executive function
- Anhedonia (loss of pleasure)
- Catatonia (reduced movement)
Cognitive symptoms
- Memory
- Attention
- Planning, decision-making
- Social cognition
- Movement
Affective dysregulation
- Depressive, manic states
Biological bases
- Genetic predisposition
- Brain abnormalities
- Developmental origins
Genetic disposition
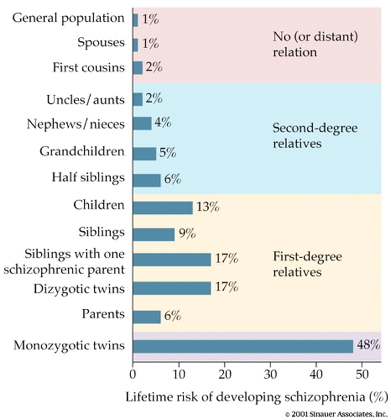
Heritability
- 80%
- vs. 60% for osteoarthritis
- 30-50% for hypertension (Os & Kapur, 2009)
But, no single gene…
Genes associated with schizophrenia at higher than chance levels
- NOTCH4, TNF:
- Part of major histocompatibility complex (MHC), cell membrane specializations involved in the immune system
- DRD2 (dopamine D2 receptor), KCNN3 (Ca+ activated K+ channel), GRM3 (metabotropic glutamate receptor)
Ventricles larger, esp in males
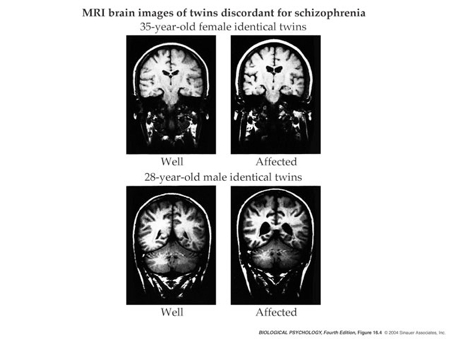
Ventricular enlargement increases across time
Enlargement precedes diagnosis?
Hip, amyg, thal, NA smaller
- Related to ventricular enlargement
- Early disturbance in brain development?
(Jiao et al., 2017)
- Dentate gyrus (DG) in hippocampus
- spatial coding, learning & memory, emotion processing
- DG dysfunction implicated in schizophrenia
- Gene linked to schizophrenia, Transmembrane protein 108 (Tmem108) enriched in DG granule neurons
- Tmem108 expression increased during postnatal period critical for DG development.
(Jiao et al., 2017)
- Tmem108-deficient neurons form fewer and smaller spines.
- Tmem108-deficient mice display schizophrenia-relevant behavioral deficits.
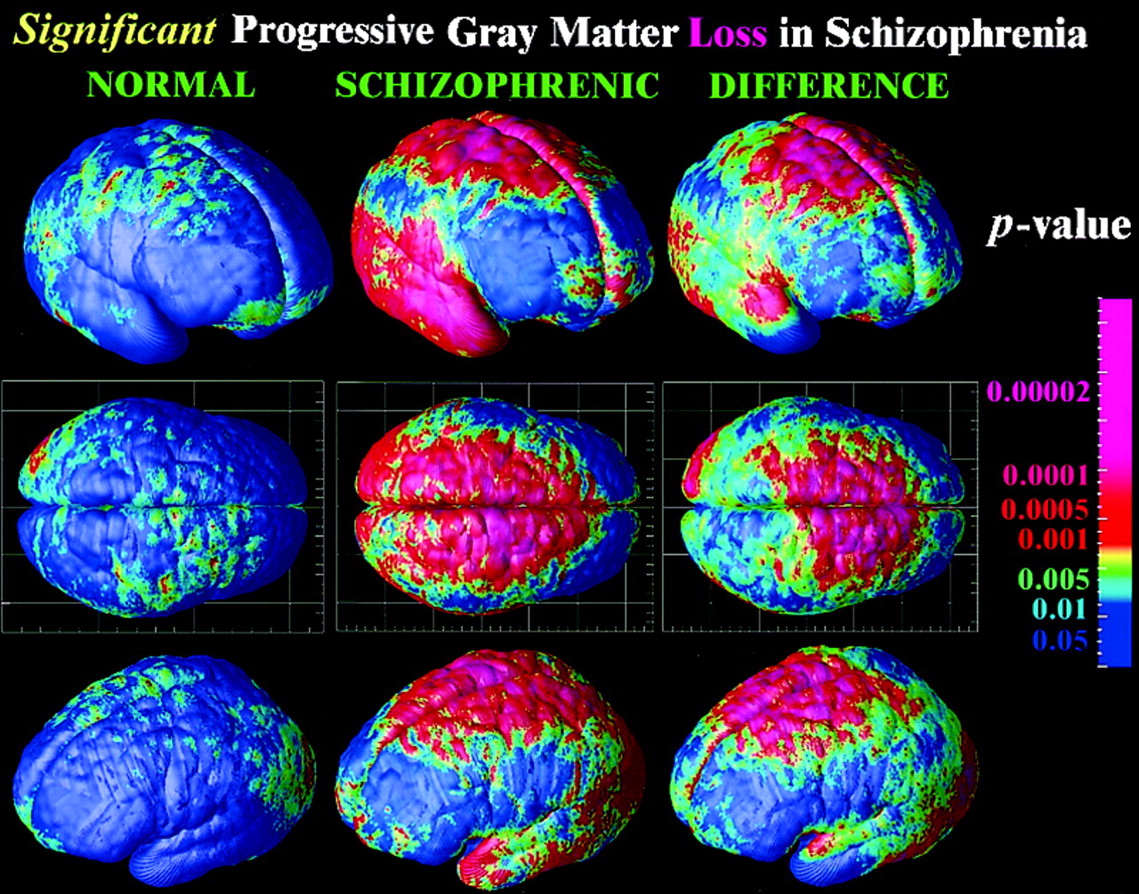
Rapid gray matter loss in adolescents?
Widespread white matter disruption
White matter loss over age
Dysconnectivity in cortical networks
Inconsistent connectivity findings (Fornito & Bullmore, 2015)
-
Structural connectivity vs.
- Synaptic, dendritic, axonal connections b/w regions
- Usually measured via DTI or related diffusion-based MRI technique
-
Functional connectivity
- BOLD, EEG, or MEG covariance
- Task-free 'resting' state or task-based
- Global signal variations?
(Fornito & Bullmore, 2015)
Global signal alterations
Dysconnectivity b/w 'hubs' -> higher functional connectivity
Dopamine hypothesis
Evidence for DA hypothesis
- DA (\(D_2\) receptor) antagonists (e.g. chlorpromazine)
- improve positive symptoms
- Typical antipsychotics are DA \(D_2\) antagonists
- DA agonists
- amphetamine, cocaine, L-DOPA
- mimic or exacerbate symptoms
Evidence against…
- New, atypical antipsychotics
- (e.g. Clozapine) INCREASE DA in frontal cortex, affect 5-HT
- Mixed evidence for high DA metabolite levels in CSF
- Some DA neurons may release 5-HT, cannabinoids, glutamate (Seutin, 2005)
Glutamate/ketamine hypothesis
- Psychomimetic drugs induce schizophrenia-like states
- Phencyclidine (PCP), ketamine
- NMDA receptor antagonists
Ketamine
- dissociative (secondary) anesthetic
- side effects include hallucinations, blurred vision, delirium, floating sensations, vivid dreams
- binds to serotonin (\(5HT_{2a}\)) receptor, \(\kappa\) opioid receptor, and \(\sigma\) receptor "chaperone"
- may be dopamine \(D_2\) receptor antagonist
Glutamate/ketamine hypothesis
- Schizophrenia == underactivation of NMDA receptors?
- NMDA receptor role in learning, plasticity
- DG neurons in (Jiao et al., 2017) were glutamate-releasing.
- NMDAR antagonists -> neurodegeneration, excitotoxicity, & apoptosis
Schizophrenia summed up
- Wide-ranging disturbance of mood, thought, action, perception
- Broad changes in brain structure, function, chemistry, development
Dopamine hypothesisgiving way to glutamate hypothesis- Genetic (polygenic = multiple genes) risk + environmental factors
Early life stress increases risk
- 2x greater odds for children in urban environments
- Higher risk among migrant populations (Cantor-Graae & Selten, 2005)
- Exposure to infection in utero, other birth complications
- Exposure to cannibis
- Paternal age > 40
(Levine, Levav, Pugachova, Yoffe, & Becher, 2016)
- Children (N=51,233) of parents who born during Nazi era (1922-1945)
- Emigrated before (indirect exposure) or after (direct exposure) to Nazi era
- Children exposed to direct stress of Nazi era in utero or postnatally
- Did not differ in rates of schizophrenia, but
- Had higher rehospitalization rates
(Debost et al., 2015)
- Danish cohort (n=1,141,447)
- Exposure to early life stress
- in utero did not increase risk of schizophrenia, but
- during 0-2 years increased risk
- Increased risk associated with an allele of a cortisol-related gene
The future: Outcomes following hospitalization
The future of psychiatric research
- The Research Domain Criteria (RDoC) Project
- Negative valence, positive valence, cognitive systems, social processes, arousal/regulatory systems
The future of psychiatric research
Next time…
- Affective disorders
References
Cantor-Graae, E., & Selten, J.-P. (2005). Schizophrenia and migration: A meta-analysis and review. The American Journal of Psychiatry, 162(1), 12–24. https://doi.org/10.1176/appi.ajp.162.1.12
Debost, J.-C., Petersen, L., Grove, J., Hedemand, A., Khashan, A., Henriksen, T., … Mortensen, P. B. (2015). Investigating interactions between early life stress and two single nucleotide polymorphisms in HSD11B2 on the risk of schizophrenia. Psychoneuroendocrinology, 60, 18–27. https://doi.org/10.1016/j.psyneuen.2015.05.013
Erp, T. G. M. van, Hibar, D. P., Rasmussen, J. M., Glahn, D. C., Pearlson, G. D., Andreassen, O. A., … Turner, J. A. (2015). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry. https://doi.org/10.1038/mp.2015.63
Fornito, A., & Bullmore, E. T. (2015). Reconciling abnormalities of brain network structure and function in schizophrenia. Curr. Opin. Neurobiol., 30, 44–50. https://doi.org/10.1016/j.conb.2014.08.006
Jiao, H.-F., Sun, X.-D., Bates, R., Xiong, L., Zhang, L., Liu, F., … Mei, L. (2017). Transmembrane protein 108 is required for glutamatergic transmission in dentate gyrus. Proceedings of the National Academy of Sciences, 114(5), 1177–1182. https://doi.org/10.1073/pnas.1618213114
Johnson, E. C., Border, R., Melroy-Greif, W. E., Leeuw, C. A. de, Ehringer, M. A., & Keller, M. C. (2017). No evidence that schizophrenia candidate genes are more associated with schizophrenia than noncandidate genes. Biol. Psychiatry, 82(10), 702–708. https://doi.org/10.1016/j.biopsych.2017.06.033
Kelly, S., Jahanshad, N., Zalesky, A., Kochunov, P., Agartz, I., Alloza, C., … Donohoe, G. (2017). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Mol. Psychiatry. https://doi.org/10.1038/mp.2017.170
Kempton, M. J., Stahl, D., Williams, S. C. R., & DeLisi, L. E. (2010). Progressive lateral ventricular enlargement in schizophrenia: A meta-analysis of longitudinal MRI studies. Schizophr. Res., 120(1-3), 54–62. https://doi.org/10.1016/j.schres.2010.03.036
Kochunov, P., Ganjgahi, H., Winkler, A., Kelly, S., Shukla, D. K., Du, X., … Hong, L. E. (2016). Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum. Brain Mapp., 37(12), 4673–4688. https://doi.org/10.1002/hbm.23336
Levine, S. Z., Levav, I., Pugachova, I., Yoffe, R., & Becher, Y. (2016). Transgenerational effects of genocide exposure on the risk and course of schizophrenia: A population-based study. Schizophrenia Research, 176(2), 540–545. https://doi.org/10.1016/j.schres.2016.06.019
Os, J. van, & Kapur, S. (2009). Schizophrenia. The Lancet, 374(9690), 635–645. https://doi.org/10.1016/S0140-6736(09)60995-8
Seutin, V. (2005). Dopaminergic neurones: Much more than dopamine? Br. J. Pharmacol., 146(2), 167–169. https://doi.org/10.1038/sj.bjp.0706328
Thompson, P. M., Vidal, C., Giedd, J. N., Gochman, P., Blumenthal, J., Nicolson, R., … Rapoport, J. L. (2001). Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proceedings of the National Academy of Sciences, 98(20), 11650–11655. https://doi.org/10.1073/pnas.201243998
Uhlhaas, P. J. (2013). Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol., 23(2), 283–290. https://doi.org/10.1016/j.conb.2012.11.004
Yang, G. J., Murray, J. D., Repovs, G., Cole, M. W., Savic, A., Glasser, M. F., … Anticevic, A. (2014). Altered global brain signal in schizophrenia. Proc. Natl. Acad. Sci. U. S. A., 111(20), 7438–7443. https://doi.org/10.1073/pnas.1405289111