- Depression
- Planning for student-led presentations
2018-11-08 16:27:54
Today's topic(s)
Depression
- Symptoms
- Unhappy mood, insomnia, lethargy, loss of pleasure, interest, energy
- Agitation
- Lasting for several weeks or more
Depression
- Experienced by ~7% Americans in any year
- Prevalence (up to ~20% lifetime)
- Females 2-3x males, higher 40+ years of age
- postpartum anxiety and depression in 10-20% of mothers
- MZ concordance ~60% vs. DZ ~20% suggests genetic component
Symptoms, (Mahar, Bambico, Mechawar, & Nobrega, 2014)
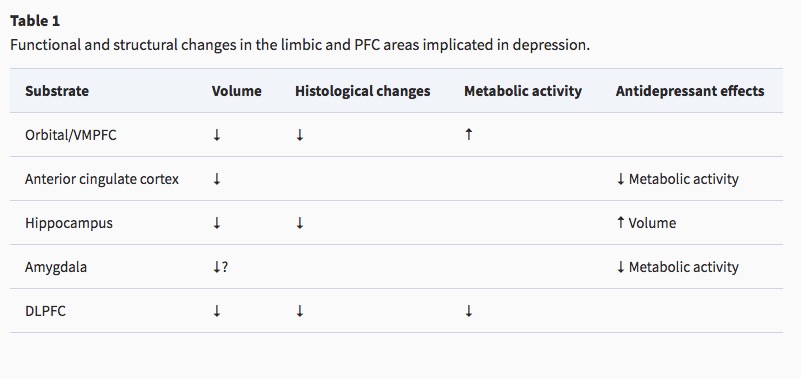
Neurobiology of Major Depressive Disorder (MDD)
- Reduced sizes of brain regions
- Hypoactivity
- Pharmacological factors
- Synaptic neurotrophic dysfunction
Neurological factors
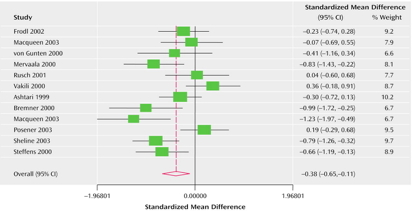
- Reduced hippocampal volumes
- (Videbech & Ravnkilde, 2004a) meta-analysis
(Videbech & Ravnkilde, 2004a)
Left Hippocampus
(Videbech & Ravnkilde, 2004b)
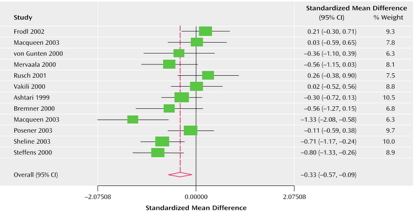
Right Hippocampus
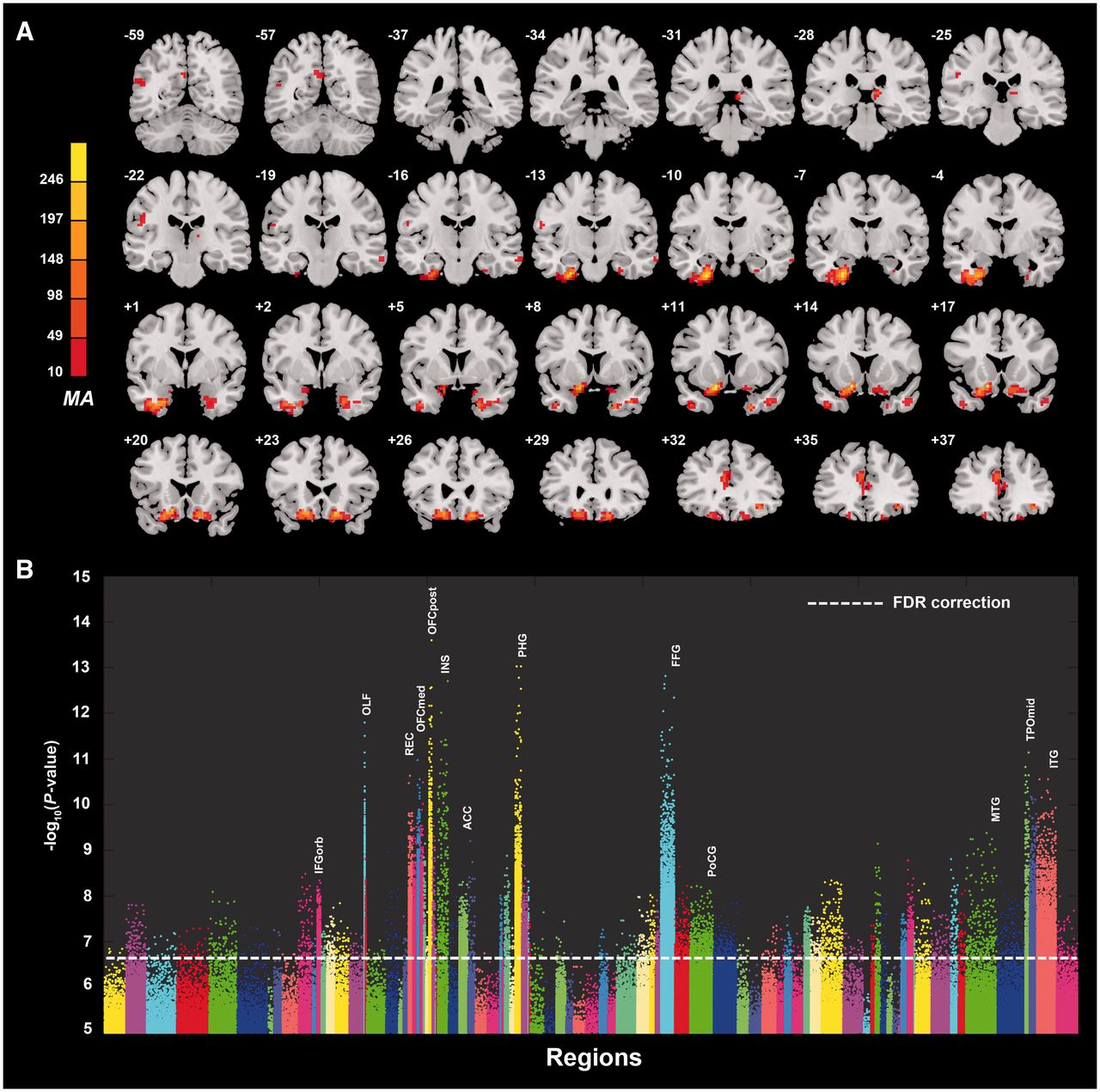
Hypoactivity in
- Frontal and temporal cortex
- Anterior cingulate
- Insula
- Cerebellum
- (Fitzgerald, Laird, Maller, & Daskalakis, 2008)
(Fitzgerald et al., 2008)
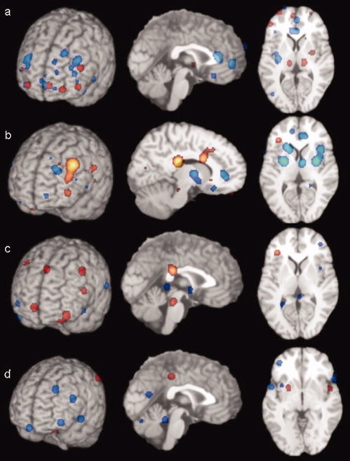
[a] patients v. ctrls, [b] patients on SSRIs, [c] patients v. ctrls (happy stim), [d] patients v. controls (sad stim)
Baseline hyperactivity (Hamilton et al., 2012)
Valence-specific hyperactivity (Hamilton et al., 2012)
Increased connectivity between resting state network regions and dorsal PFC (Sheline, Price, Yan, & Mintun, 2010)
Altered connectivity
- Resting state fMRI (rsFMRI) in 421 patients with major depressive disorder and 488 control subjects.
- Reduced connectivity between orbitofrontal cortex (OFC) and other areas of the brain
- Increased connectivity between lateral PFC and other brain areas
Pharmacological factors
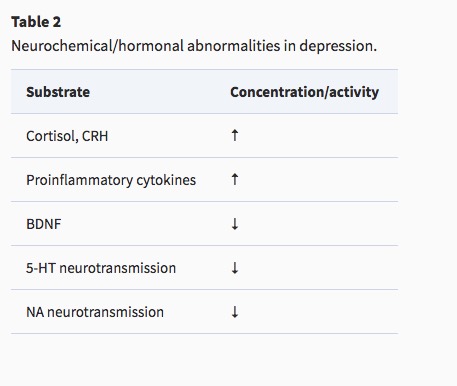
- Endocrine
- Thyroid dysfunction (Medici et al., 2014)
- Altered cortisol reactivity (Burke, Davis, Otte, & Mohr, 2005)
Pharmacological factors
- Monoamine hypothesis
- More: euphoria
- Less: depression
- Resperine (antagonist for NE & 5-HT) can cause depression
- Low serotonin (5-HT) metabolite levels in CSF of suicidal depressives (Samuelsson, Jokinen, Nordström, & Nordström, 2006)
Measuring 5-HT
- CSF, platelets, plasma, urine, saliva
- CSF & platelets correlate highly (Audhya, Adams, & Johansen, 2012)
- Salivary 5-HT does not correlate with mood symptoms (Leung et al., 2018)
Drug treatments
- Monoamine oxidase (MAO) inhibitors
- MAO inactivates monoamines in terminal buttons
- MAO-I’s boost monoamine levels
- Tricyclics
- Inhibit NE, 5-HT reuptake
- Upregulate monoamine levels, but non-selective = side effects
Drug treatments
- Selective Serotonin Reuptake Inhibitors (SSRIs)
- Fluoxetine (Prozac, Paxil, Zoloft)
- Prolong duration 5-HT in synaptic cleft
- Also increase brain steroid production
- Serotonin/Norepinephrine Reuptake Inhibitors (SNRIs)
Cymbalta (SNRI)
How well do drugs work?
- STAR*D trial
- On SSRI for 12-14 weeks. ~1/3 achieved remission; 10-15% showed symptom reduction.
- If SSRI didn't work, could switch drugs. ~25% became symptom free.
- 16% of participants dropped out due to tolerability issues
- Took 6-7 weeks to show response.
Who benefits from drug therapy?
- Depends on
- Early life stress
- Brain (amygdala) response to emotional faces (Goldstein-Piekarski et al., 2016)
- Low-stress + low amyg reactivity -> > responding
- High stress + high amyg reactivity -> > responding
Problems with monoamine hypothesis
- Too simplistic
- NE, 5-HT interact
- Drugs fast acting (min), but improvement slow (weeks)
- "No correlation between serotonin and its metabolite 5-HIAA in the cerebrospinal fluid and [11C]AZ10419369 binding measured with PET in healthy volunteers". (Tiger et al., 2015)
"…we performed the first meta-analysis of the mood effects in ATD and APTD studies. The depletion of monoamine systems (both 5-HT and NE/DA) does not decrease mood in healthy controls. However, in healthy controls with a family history of MDD the results suggest that mood is slightly decreased…by [monoamine depletion]…"
What do drugs do, then?
Ketamine again
- Relieves depressive symptoms relatively quickly (Berman et al., 2000) and (Zarate et al., 2006)
- Boosts synaptic spine formation (N. Li et al., 2010) and reverses effects of induced stress
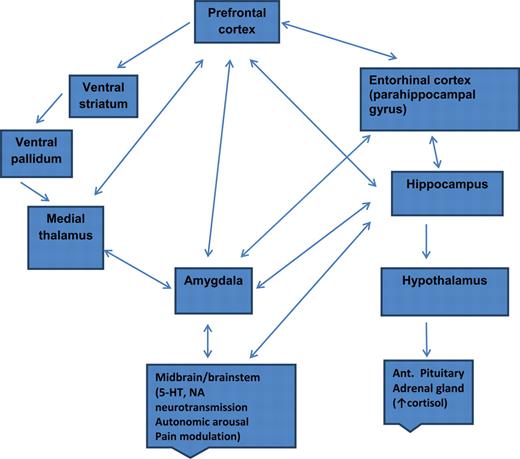
Pathway of pathology (Duman & Aghajanian, 2012)
- Depression ~ chronic stress (Mahar et al., 2014)
- Stress -> chronic HPA axis activity
- Chronic HPA activity -> neuronal atrophy in hipp & PFC
- Stress & cortisol decrease expression of brain-derived neurotrophic factor (BDNF)
- BDNF boosts neurogenesis
- SSRIs act via BDNF, as do NMDA receptor antagonists (e.g., ketamine)
(Duman & Voleti, 2012)
(Frohlich & Van Horn, 2014)
Electroconvulsive Therapy (ECT)
- Last line of treatment for drug-resistant depression
- Electric current delivered to the brain causes 30-60s seizure.
- ECT usually done in a hospital's operating or recovery room under general anesthesia.
- Once every 2 - 5 days for a total of 6 - 12 sessions.
Electroconvulsive Therapy (ECT)
- Remission rates of up to 50.9% (Dierckx, Heijnen, Broek, & Birkenhäger, 2012)
- Seems to work via
- Anticonvulsant (block Na+ channel or enhance GABA function) effects
- Neurotrophic (stimulates neurogenesis) effects
Putting the pieces together
The disordered mind: Take home messages
- Multi-level, multi-method, multi-variate approaches essential to understanding mental illness
- Developmental processes across the life span
- Networks all the way down…
References
Audhya, T., Adams, J. B., & Johansen, L. (2012). Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochimica et Biophysica Acta, 1820(10), 1496–1501. https://doi.org/10.1016/j.bbagen.2012.05.012
Berman, R. M., Cappiello, A., Anand, A., Oren, D. A., Heninger, G. R., Charney, D. S., & Krystal, J. H. (2000). Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry, 47(4), 351–354. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10686270
Burke, H. M., Davis, M. C., Otte, C., & Mohr, D. C. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. https://doi.org/10.1016/j.psyneuen.2005.02.010
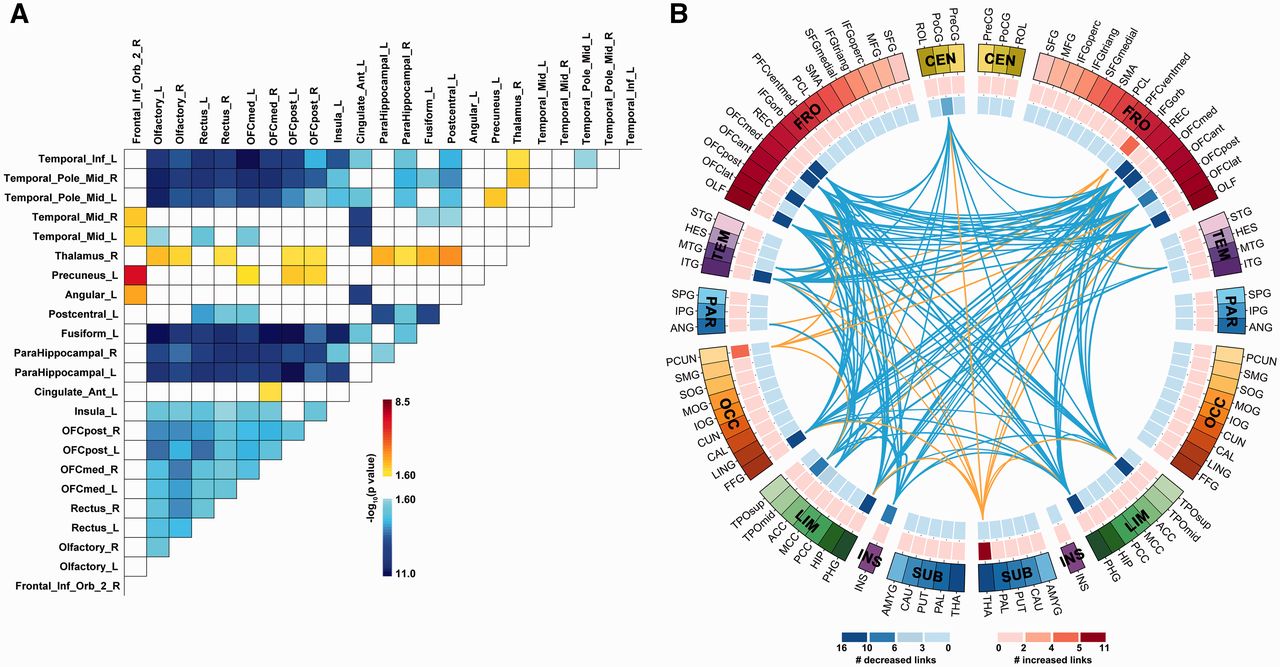
Cheng, W., Rolls, E. T., Qiu, J., Liu, W., Tang, Y., Huang, C.-C., … Feng, J. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, aww255. https://doi.org/10.1093/brain/aww255
Dierckx, B., Heijnen, W. T., Broek, W. W. van den, & Birkenhäger, T. K. (2012). Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: A meta-analysis. Bipolar Disorders, 14(2), 146–150. https://doi.org/10.1111/j.1399-5618.2012.00997.x
Duman, R. S., & Aghajanian, G. K. (2012). Synaptic dysfunction in depression: Potential therapeutic targets. Science, 338(6103), 68–72. https://doi.org/10.1126/science.1222939
Duman, R. S., & Voleti, B. (2012). Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci., 35(1), 47–56. https://doi.org/10.1016/j.tins.2011.11.004
Fitzgerald, P. B., Laird, A. R., Maller, J., & Daskalakis, Z. J. (2008). A meta-analytic study of changes in brain activation in depression. Human Brain Mapping, 29(6), 683–695. https://doi.org/10.1002/hbm.20426
Frohlich, J., & Van Horn, J. D. (2014). Reviewing the ketamine model for schizophrenia. J. Psychopharmacol., 28(4), 287–302. https://doi.org/10.1177/0269881113512909
Goldstein-Piekarski, A. N., Korgaonkar, M. S., Green, E., Suppes, T., Schatzberg, A. F., Hastie, T., … Williams, L. M. (2016). Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proceedings of the National Academy of Sciences, 113(42), 11955–11960. https://doi.org/10.1073/pnas.1606671113
Hamilton, J. P., Etkin, A., Furman, D. J., Lemus, M. G., Johnson, R. F., & Gotlib, I. H. (2012). Functional neuroimaging of major depressive disorder: A Meta-Analysis and new integration of baseline activation and neural response data. AJP, 169(7), 693–703. https://doi.org/10.1176/appi.ajp.2012.11071105
Leung, J., Selvage, C., Bosdet, T., Branov, J., Rosen-Heath, A., Bishop, C., … Horvath, G. (2018). Salivary serotonin does not correlate with central serotonin turnover in adult phenylketonuria (PKU) patients. Molecular Genetics and Metabolism Reports, 15, 100–105. https://doi.org/10.1016/j.ymgmr.2018.03.008
Li, N., Lee, B., Liu, R.-J., Banasr, M., Dwyer, J. M., Iwata, M., … Duman, R. S. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329(5994), 959–964. https://doi.org/10.1126/science.1190287
Mahar, I., Bambico, F. R., Mechawar, N., & Nobrega, J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neuroscience & Biobehavioral Reviews, 38, 173–192. https://doi.org/10.1016/j.neubiorev.2013.11.009
Medici, M., Direk, N., Visser, W. E., Korevaar, T. I. M., Hofman, A., Visser, T. J., … Peeters, R. P. (2014). Thyroid function within the normal range and the risk of depression: A population-based cohort study. J. Clin. Endocrinol. Metab., 99(4), 1213–1219. https://doi.org/10.1210/jc.2013-3589
Palazidou, E. (2012). The neurobiology of depression. British Medical Bulletin, 101, 127–145. https://doi.org/10.1093/bmb/lds004
Ruhé, H. G., Mason, N. S., & Schene, A. H. (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Molecular Psychiatry, 12(4), 331–359. https://doi.org/10.1038/sj.mp.4001949
Samuelsson, M., Jokinen, J., Nordström, A.-L., & Nordström, P. (2006). CSF 5-HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatrica Scandinavica, 113(1), 44–47. https://doi.org/10.1111/j.1600-0447.2005.00639.x
Sheline, Y. I., Price, J. L., Yan, Z., & Mintun, M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A., 107(24), 11020–11025. https://doi.org/10.1073/pnas.1000446107
Tiger, M., Svenningsson, P., Nord, M., Jabre, S., Halldin, C., & Lundberg, J. (2015). No correlation between serotonin and its metabolite 5-HIAA in the cerebrospinal fluid and [11C]AZ10419369 binding measured with PET in healthy volunteers. Retrieved from http://hdl.handle.net/10616/44513
Videbech, P., & Ravnkilde, B. (2004a). Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry, 161(11), 1957–1966. https://doi.org/10.1176/appi.ajp.161.11.1957
Videbech, P., & Ravnkilde, B. (2004b). Hippocampal volume and depression: A meta-analysis of mri studies. American Journal of Psychiatry, 161(11), 1957–1966. https://doi.org/10.1176/appi.ajp.161.11.1957
Zarate, C. A., Jr, Singh, J. B., Carlson, P. J., Brutsche, N. E., Ameli, R., Luckenbaugh, D. A., … Manji, H. K. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry, 63(8), 856–864. https://doi.org/10.1001/archpsyc.63.8.856