PSY 511
Evolution and development of the human brain
Rick Gilmore
2021-10-14 08:18:35
- Fun
- Evolution
- Human brain development
- Timeline of milestones
- Prenatal period
- Differentiation
- Infancy & Early Childhood
- Summary of developmental milestones
- How brain development clarifies anatomical structure
- References
Fun
Evolution
Public acceptance of evolution
- In U.S., majority now “accept”
- Increase over last decade
Types of evidence
- Fossil
- Fossil dating
- Geological
- Where fossils are found relative to one another
- How long it takes to form layers
- Genetic
- Rates of mutation
- Anatomical
- Homologous structures across species
Nothing in Biology Makes Sense except in the Light of Evolution
“Seen in the light of evolution, biology is, perhaps, intellectually the most satisfying and inspiring science. Without that light, it becomes a pile of sundry facts some of them interesting or curious, but making no meaningful picture as a whole.”
Why Gilmore thinks the theory so controversial (in the U.S.)
- Contradicts verbatim/non-metaphorical reading of some religious texts
- Makes humans seem less special
- Time scales involved beyond human experience
- Scientific method vs. other ways of knowing
- Found in nature ≠ good for human society
- Few negative consequences of ‘disbelief’
- U.S. culture individualistic, skeptical, anti-elitist, anti-intellectual
- Lower levels of religious belief among U.S. scientists
- Politics
- A minority of citizens support teaching evolution-only
- Majority of classroom teachers aren’t strong advocates
A structural equation model indicates that increasing enrollment in baccalaureate-level programs, exposure to college-level science courses, a declining level of religious fundamentalism, and a rising level of civic scientific literacy are responsible for the increased level of public acceptance.
Evolution and development
Ontogenesis and phylogenesis
- Ontogenesis
- Development within lifetimes, history of individuals
- Phylogenesis
- Change across lifestimes, history of species
Ontogeny does not recapitulate phylogeny (Haeckel), but…

Source: Wikipedia
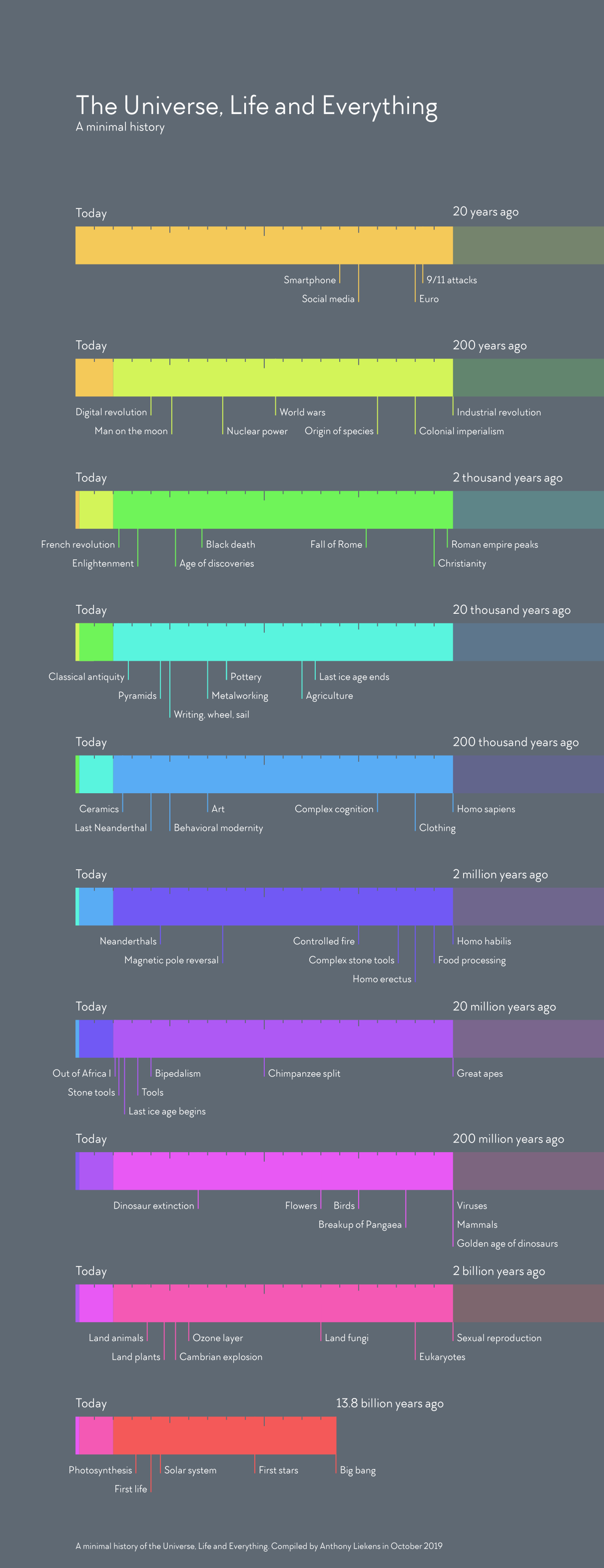
Complex multicellular life emerged “recently”
Nervous system architectures
How nervous systems differ
- Body symmetry
- radial
- bilateral
An animal with a nerve “net”
- Segmentation
- Cephalization (concentration of sensory & neural structures in anterior portion of body)
- Encasement in bone (vertebrates)
- Centralized vs. distributed function
Cephalopods have “intelligent arms”
The essentials of biological computation
- Ingestion
- Defense
- Reproduction
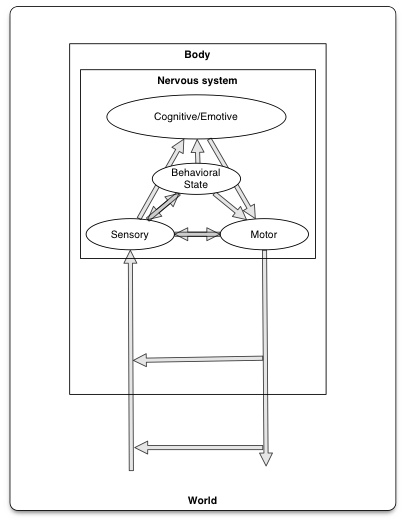
Information processing universals
- Sense/detect via sensors
- Specialize by information source/type
- Specialize by target location
- Interoceptive
- Exteroceptive
- Analyze, evaluate, decide
- Current state
- World
- Organism
- Current goals
- Past state(s)
- Current state
- Act
- Move body
- Approach/avoid
- Manipulate
- Ingest
- Signal
- Change physiological state
- Move body
From nerve net to nerve ring, nerve cord, and brain
(Arendt, Tosches, & Marlow, 2016)
Figure 1: Animal phylogeny. A simplified animal phylogenetic tree (showing the evolutionary history of animals), in which lines represent evolutionary diversification. The lengths of the lines are arbitrary, as they do not indicate evolutionary distance. For a brief characterization of the Anthozoa, Bilateria, Ceriantharia, Cnidaria, Ctenophora, Medusozoa and Neuralia, see the glossary. The phylogenetic position of the Ctenophora is not settled, as indicated by a question mark. The Ctenophora image is adapted with permission from Ref. 43, Wiley. The Porifera and Placozoa images are reprinted with permission from Ref. 139 (Nielsen, C. Animal Evolution: Interrelationships of the Living Phyla p31 and p39 (2012)) by the permission of Oxford University Press. The Annelida image is adapted with permission from Ref. 140, Schweizerbart Science Publishers (www.schweizerbart.de).
Figure 2: Comparison of neurodevelopment in the frog, annelid and sea anemone. The frog Xenopus laevis (part a), the annelid Platynereis dumerilii (part b) and the cnidarian Nematostella vectensis (part c) are depicted in their gastrula-like stages (gastrula, trochophora and planula, respectively; upper panels), intermediate developmental (neurula, metatrochophora and late planula, respectively; middle panels) and juvenile stages (tadpole, nectochaete and polyp, respectively; lower panels). Colours demarcate developmental neurogenic regions, and double-headed arrows show the apical (AP)–blastoporal (BL) axis. All views in parts a–c are lateral. At gastrula stages (upper panels), blastoporal ectodermal tissue (around the closing blastopore; red) and apical pole ectodermal tissue (violet) can be distinguished. At subsequent stages, a large part of the ectoderm — incluing the former apical and blastoporal regions — gives rise to neurogenic tissue. The neurogenic tissue comprises regions of distinct molecular identity (indicated by different colours), which will give rise to different parts of the nervous system. In the frog (part a), the neural plate (violet, red and yellow) comprises future forebrain tissue, as well as medial and lateral neural tube tissue; it is laterally bounded by developing peripheral nervous system components (blue). Similar regions are apparent in the annelid (part b), and these give rise to the brain, medial and lateral nerve cord and peripheral nervous system. As reasoned in this article, these regions also exist in the cnidarian (part c). In the frog and annelid worm, these regions are further subdivided into specific subregions by the activity of molecular organizing signals15.
- Neurons and nervous systems 520-570 M years old
- Diverse nervous systems show developmental similarities at molecular level
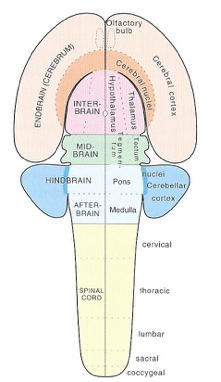
Vertebrate CNS organization
- Differences in size of the cerebral cortex
| Structural measure | Non-human comparison | Human |
|---|---|---|
| Cortical gray matter %/tot brain vol | insectivores 25% | 50% |
| Cortical gray + white | mice 40% | 80% |
| Cerebellar mass | primates, mammals 10-15% | 10-15% |
- Evidence for greater gray and white matter (relative to total brain volume) in human cerebral cortex
Take homes
- Brain sizes scale with body size
- Brain sizes (more or less) scale with animal class (more or less)
Old story
- Within mammals, human brains bigger than expected
- Higher encephalization quotient – deviation from species-typical norm
- Humans have larger cerebral cortical gray + white matter than comparable mammals
vs. New story
- Does brain size/mass matter (that much)?
- “Size matters” (brain mass) presumes similarity among brains at micro-level
- Big (large mass) brains arise in multiple mammalian lineages
- # of cortical neurons more important difference than brain mass
- The primate advantage -> more cortical neurons, but not larger neurons & not more neurons in cerebellum
- Human brain just scaled up (non-ape) primate brain
# of cortical (or in birds, pallidum) neurons predicts “cognition?”
The Human Advantage (Herculano-Houzel, 2016)
- Brain
- More neurons in cerebral cortex than other mammals
- Behavior
- Less time spent foraging
- Higher quality/more energetically dense food
- Higher food availability
- Cultural factors (agriculture + cooking), see also (Wrangham, 2009)
- Less time spent foraging
A further human advantage

![[[@miller2006public]](http://dx.doi.org/10.1126/science.1126746)](https://www.science.org/cms/10.1126/science.1126746/asset/04dcf902-b601-44d0-8a9c-fd7f717b57a3/assets/graphic/765-1.gif)
![[[@Miller2021-lb]](http://dx.doi.org/10.1177/09636625211035919)](https://journals-sagepub-com.ezaccess.libraries.psu.edu/na101/home/literatum/publisher/sage/journals/content/pusa/0/pusa.ahead-of-print/09636625211035919/20210815/images/medium/10.1177_09636625211035919-fig1.gif)
![[[@arendt_nerve_2016]](http://doi.org/10.1038/nrn.2015.15)](img/nrn.2015.15-f1.jpg)
![[[@arendt_nerve_2016]](http://doi.org/10.1038/nrn.2015.15)](img/nrn.2015.15-f2.jpg)
![[[@Northcutt2002-jg]](http://doi.org/10.1093/icb/42.4.743)](img/northcutt-2002-f1.gif)


![[[@Northcutt2002-jg]](http://doi.org/10.1093/icb/42.4.743)](img/northcutt-2002-f2.gif)
![[[@hofman_evolution_2014]](https://doi.org/10.3389/fnana.2014.00015)](http://www.frontiersin.org/files/Articles/78485/fnana-08-00015-HTML/image_m/fnana-08-00015-g001.jpg)
![[[@rakic2009evolution]](http://dx.doi.org/10.1038/nrn2719)](https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fnrn2719/MediaObjects/41583_2009_Article_BFnrn2719_Fig1_HTML.jpg?as=webp)
![[[@hofman_evolution_2014]](https://doi.org/10.3389/fnana.2014.00015)](http://www.frontiersin.org/files/Articles/78485/fnana-08-00015-HTML/image_m/fnana-08-00015-g002.jpg)
![[[@Herculano-Houzel2012-up]](http://doi.org/10.1073/pnas.1201895109)](http://www.pnas.org/content/109/Supplement_1/10661/F1.large.jpg)
![[[@Herculano-Houzel2012-up]](http://doi.org/10.1073/pnas.1201895109)](http://www.pnas.org/content/109/Supplement_1/10661/F3.large.jpg)
![[[@Herculano-Houzel2017-gf]](http://doi.org/10.1016/j.cobeha.2017.02.004)](https://ars.els-cdn.com/content/image/1-s2.0-S2352154616302637-gr3_lrg.jpg)
![[[@Silbereis2016-la]](http://dx.doi.org/10.1016/j.neuron.2015.12.008)](https://ars.els-cdn.com/content/image/1-s2.0-S0896627315010806-gr1_lrg.jpg)

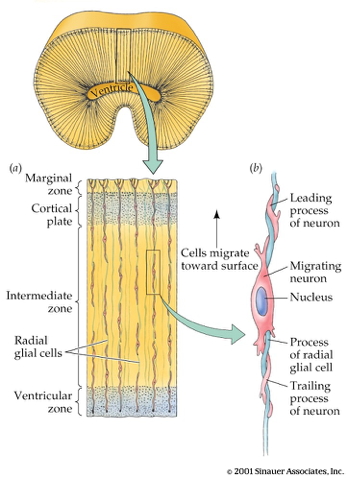
![[[@Gotz2005-yj]](https://doi.org/10.1038/nrm1739)](https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fnrm1739/MediaObjects/41580_2005_Article_BFnrm1739_Fig1_HTML.jpg?as=webp)
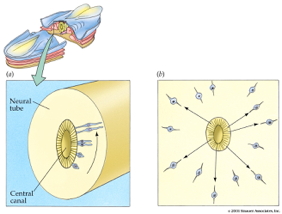
![[[@rakic2009evolution]](http://dx.doi.org/10.1038/nrn2719)](http://www.nature.com/nrn/journal/v10/n10/images/nrn2719-f2.jpg)
![[[@Baumann2001-nw]](http://dx.doi.org/10.1152/physrev.2001.81.2.871)](https://www.physiology.org/na101/home/literatum/publisher/physio/journals/content/physrev/2001/physrev.2001.81.issue-2/physrev.2001.81.2.871/production/images/medium/9j0210133004.jpeg)
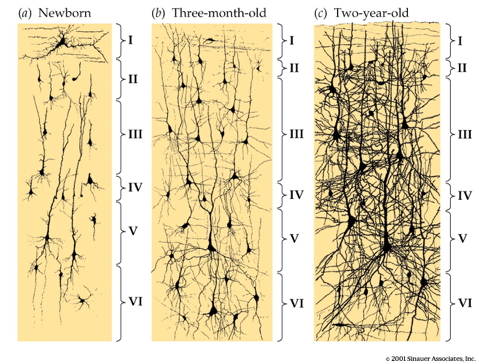
![[[@rakic2009evolution]](http://dx.doi.org/10.1038/nrn2719)](http://www.nature.com/nrn/journal/v10/n10/images/nrn2719-f3.jpg)
![[[@Baumann2001-nw]](http://dx.doi.org/10.1152/physrev.2001.81.2.871)](https://www.physiology.org/na101/home/literatum/publisher/physio/journals/content/physrev/2001/physrev.2001.81.issue-2/physrev.2001.81.2.871/production/images/medium/9j0210133006.jpeg)
![[[@Chi1977-hm]](http://doi.org/10.1002/ana.410010109)](img/chi-77-1.jpg)
![[[@Chi1977-hm]](http://doi.org/10.1002/ana.410010109)](img/chi-77-2.jpg)
![[[@Chi1977-hm]](http://doi.org/10.1002/ana.410010109)](img/chi-77-3.jpg)
![[[@Chi1977-hm]](http://doi.org/10.1002/ana.410010109)](img/chi-77-4.jpg)

![[[@Hagmann02112010]](http://doi.org/10.1073/pnas.1009073107)](http://www.pnas.org/content/107/44/19067/F1.medium.gif)
![[[@irimia_2014]](http://doi.org/10.3389/fnhum.2014.00051)](https://pressroom.usc.edu/files/2014/02/brain-networks.jpg)
![[[@Petrican2017-re]](http://doi.org/10.1016/j.neuroimage.2017.09.025)](https://ars.els-cdn.com/content/image/1-s2.0-S1053811917307735-gr2a_lrg.jpg)
![[[@Petrican2017-re]](http://doi.org/10.1016/j.neuroimage.2017.09.025)](https://ars.els-cdn.com/content/image/1-s2.0-S1053811917307735-gr2c_lrg.jpg)
![[[@Cao2017-bl]](http://doi.org/10.1016/j.tins.2017.06.003)](https://ars.els-cdn.com/content/image/1-s2.0-S0166223617301157-gr2_lrg.jpg)
![[[@Hagmann02112010]](http://doi.org/10.1073/pnas.1009073107)](http://www.pnas.org/content/107/44/19067/F2.medium.gif)
![[[@Shaw2008-dq]](https://doi.org/10.1523/JNEUROSCI.5309-07.2008)](http://www.jneurosci.org/content/jneuro/28/14/3586/F1.large.jpg)
![[[@Shaw2008-dq]](https://doi.org/10.1523/JNEUROSCI.5309-07.2008)](http://www.jneurosci.org/content/jneuro/28/14/3586/F2.large.jpg)
![[[@Shaw2008-dq]](https://doi.org/10.1523/JNEUROSCI.5309-07.2008)](http://www.jneurosci.org/content/jneuro/28/14/3586/F3.large.jpg)
![[[@Shaw2008-dq]](https://doi.org/10.1523/JNEUROSCI.5309-07.2008)](http://www.jneurosci.org/content/jneuro/28/14/3586/F4.large.jpg)
![[[@Kuzawa2014-qd]](http://doi.org/10.1073/pnas.1323099111)](http://www.pnas.org/content/111/36/13010/F1.medium.gif)
![[[@Kang2011-ex]](http://doi.org/10.1038/nature10523)](https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fnature10523/MediaObjects/41586_2011_Article_BFnature10523_Fig5_HTML.jpg?as=webp)


![[[@Johnson2001-yy]](http://doi.org/10.1038/35081509)](https://media.springernature.com/full/springer-static/image/art%3A10.1038%2F35081509/MediaObjects/41583_2001_Article_BF35081509_Fig3_HTML.gif?as=webp)