2019-03-11 18:34:59
Prelude {.smaller} 2:39
Today's Topics
- Depression
- Bipolar disorder
Depression
- Symptoms
- Unhappy mood, insomnia, lethargy, loss of pleasure, interest, energy
- Agitation
- Lasting for several weeks or more
Depression
- Experienced by ~7% Americans in any year
- Prevalence (up to ~20% lifetime)
- Females 2-3x males, higher 40+ years of age
- MZ concordance ~60% vs. DZ ~20% suggests genetic component
Symptoms, (Mahar et al. 2014)
Neurobiology of Major Depressive Disorder (MDD)
- Reduced sizes of brain regions
- Hypoactivity
- Pharmacological factors
- Synaptic dysfunction
Neurological factors
- Reduced hippocampal volumes
- (Videbech and Ravnkilde 2004b) meta-analysis
(Videbech and Ravnkilde 2004b)
Left Hippocampus
(Videbech and Ravnkilde 2004a)
Right Hippocampus
Neurological factors
- Hypoactivity in
- Frontal and temporal cortex
- Anterior cingulate
- Insula
- Cerebellum
- (Fitzgerald et al. 2008)
(Fitzgerald et al. 2008)
- patients v. controls, (b) patients on SSRIs, (c) patients v. ctrls (happy stim), (d) patients v. controls (sad stim)
Baseline hyperactivity (Hamilton et al. 2012)
Valence-specific hyperactivity (Hamilton et al. 2012)
Disrupted connectivity
- Resting state fMRI (rsFMRI) in \(n=421\) patients with major depressive disorder and \(n=488\) control subjects.
- Reduced connectivity between orbitofrontal cortex (OFC) and other areas of the brain
- Increased connectivity between lateral PFC and other brain areas
Pharmacological factors
- Endocrine
- Thyroid dysfunction (Medici et al. 2014)
- Altered cortisol reactivity (Burke et al. 2005)
Pharmacological factors
- Monoamine hypothesis
- More: euphoria
- Less: depression
- Resperine (antagonist for NE & 5-HT) can cause depression
- Low serotonin (5-HT) metabolite levels in CSF of suicidal depressives (Samuelsson et al. 2006)
Measuring 5-HT
- CSF, platelets, plasma, urine, saliva
- CSF & platelets correlate highly (Audhya, Adams, and Johansen 2012)
- Salivary 5-HT does not correlate with mood symptoms (Leung et al. 2018)
Treatments for depression
- Psychotherapy
- Often effective when combined with drug treatment
- Exercise
- Drugs
Drugs
- Monoamine oxidase (MAO) inhibitors
- MAO destroys excess monoamines in terminal buttons
- MAO-I’s boost monoamine levels
- Tricyclics
- Inhibit NE, 5-HT reuptake
- Upregulate monoamine levels, but non-selective = side effects
Drugs
- Selective Serotonin Reuptake Inhibitors (SSRIs)
- Fluoxetine (Prozac, Paxil, Zoloft)
- Prolong duration of 5-HT in synaptic cleft
- Also increase brain steroid production
- Selective Serotonin Norepinephrine Reuptake Inhibitors (SNRIs)
Cymbalta (SNRI)
How well do the drugs work?
- STAR*D trial
- On SSRI for 12-14 weeks. ~1/3 achieved remission; 10-15% showed symptom reduction.
- If SSRI didn't work, could switch drugs. ~25% became symptom free.
- 16% of participants dropped out due to tolerability issues
- Took 6-7 weeks to show response.
Who will benefit from drug therapy?
- Depends on
- Early life stress
- Brain (amygdala) response to emotional faces
- (Goldstein-Piekarski et al. 2016)
- Low-stress + low amyg reactivity -> > responding
- High stress + high amyg reactivity -> > responding
Problems with monoamine hypothesis
- Too simplistic
- NE, 5-HT interact
- Drugs fast acting (min), but improvement slow (weeks)
"No correlation between serotonin and its metabolite 5-HIAA in the cerebrospinal fluid and [11C]AZ10419369 binding measured with PET in healthy volunteers." (Tiger et al. 2015)
"…we performed the first meta-analysis of the mood effects in [acute tryptophan depletion] ATD and [alpha-methyl-para-tyrosine] APTD studies. The depletion of monoamine systems (both 5-HT and NE/DA) does not decrease mood in healthy controls. However, in healthy controls with a family history of MDD the results suggest that mood is slightly decreased…by [monoamine depletion]…"
What do drugs do, then?
- Alter receptor sensitivity?
- Serotonin presynaptic autoreceptors compensate
- Postsynaptic upregulation of NE/5-HT effects
What do drugs do, then?
- Stimulate neurogenesis?
- Link to neurotrophin, brain-derived nerve growth factor (BDNF)
- BDNF boosts neurogenesis
- SSRIs stimulate growth of new neurons in hippocampus
Neurogenesis hypothesis, (Mahar et al. 2014)
- Chronic stress causes neural loss in hipp
- Chronic stress downregulates 5-HT sensitivity
- Depression ~ chronic stress
- Anti-depressants upregulate neurogenesis via 5-HT modulation
Ketamine
- Selective antagonist of the NMDA receptor, an ionotropic glutamate receptor
- Relieves depressive symptoms relatively quickly (Berman et al. 2000) and (Zarate et al. 2006)
- Boosts synaptic spine formation (Li et al. 2010) and reverses effects of induced stress
Electroconvulsive Therapy (ECT)
- Last line of treatment for drug-resistant depression
- Electric current delivered to the brain causes 30-60s seizure.
- ECT usually done in a hospital's operating or recovery room under general anesthesia.
- Once every 2 - 5 days for a total of 6 - 12 sessions.
Electroconvulsive Therapy (ECT)
- Remission rates of up to 50.9% (Dierckx et al. 2012)
- Seems to work via
- Anticonvulsant (block Na+ channel or enhance GABA function) effects
- Neurotrophic (stimulates neurogenesis) effects
Patients speak
- Kitty Dukakis' (wife of former Governor/Presidential candidate Michael Dukakis) story: http://www.nytimes.com/2016/12/31/us/kitty-dukakis-electroshock-therapy-evangelist.html
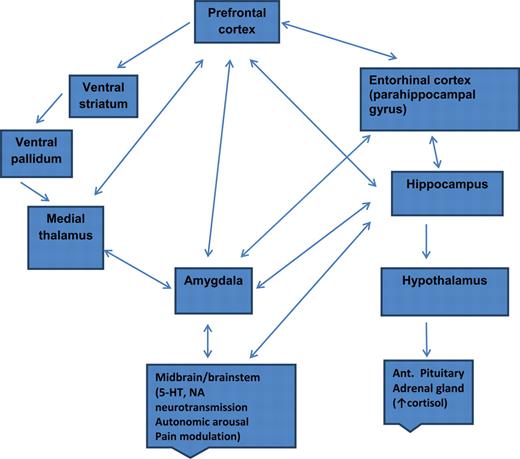
Depression's widespread impact
- Widespread brain dysfunction
- Prefrontal cortex, amygdala, HPA axis, circadian rhythms
- Genetic + environmental factors
- Disturbance in 5-HT, NE systems, cortisol
- Many sufferers do not respond to available treatments
Points on depression
- Drug treatments affect neuromodulator NT systems, but
- Can't effectively measure NT levels
- Neuromodulators interact, so many side-effects
- 'Monoamine hypothesis' of depression is at-best incomplete
- 'Talk' therapies can change behavior/mood by creating new/strengthened circuits
Major affective (emotional) disorders
- Types
- Depression
- Anxiety
- Bipolar disorder
- Heritability
- proportion of variance in trait accounted for by genetic factors
- Monozygotic: .69
- Dizygotic: .13
Bipolar disorder
- Formerly “manic depression” or “manic depressive disorder
- Alternating mood states
- Mania or hypomania (milder form)
- Depression
- Cycles 3-6 mos in length, but
- Rapid cycling (weeks or days)
- Suicide risk 20-60x normal population, (Baldessarini, Pompili, and Tondo 2006)
Symptoms
Prevalence, subtypes
- 1-3% prevalence, subthreshold affects another 2%
- Subtypes
- Bipolar I: manic episodes, possible depressive ones
- Bipolar II: no manic episodes but hypomania (disinhibition, irritability/agitation) + depression
Related symptoms
- Psychosis (hallucinations or delusions)
- Anxiety, attention-deficit hyperactivity disorder (ADHD)
- Substance abuse
Genetics
- Overlap between bipolar disorder and schizophrenia
- Genes for voltage-gated Ca++ channels
- Regulate NT, hormone release
- Gene expression, cell metabolism
- (Craddock and Sklar 2013)
Brain responses to emotional faces ≠ depression
(Lawrence et al. 2004)
(Lawrence et al. 2004)
Amyg, Hip volume reduced; ventricles larger
(Hallahan et al. 2011)
Drug treatments
- Mood stabilizers
- Lithium (Li)
- Valproate (Depakote)
- Anticonvulsants
- GABA agonists
- Usually to treat epilepsy
- e.g. lamotrigine (Lamictal)
- Atypical antipsychotics
Lithium "discovered" accidentally
- Injections of manic patients' urine with lithium compound (chemical stabilizer) into guinea pig test animals
- Had calming effect
- John Cade discovered in 1948
- Earliest effective medications for treating mental illness
Effects of Lithium
- Reduces mania, minimal effects on depressive states
- Preserves PFC, hip, amyg volume
- downregulates DA, glu; upregulates GABA
- modulates 5-HT, NE
- levels can be tested/monitored via blood test
- (Malhi et al. 2013)
Other treatment options
- Psychotherapy
- Electroconvulsive Therapy (ECT)
- Sleep medications
Prospects
- STEP-BD cohort (n=1469)
- 58% achieved recovery
- 49% had recurrences within 2 years
- Residual depressive symptoms can persist
- (Geddes and Miklowitz 2013)
An Unquiet Mind
BP summed-up
- Changes in mood, but ≠ depression
- Genetic + environmental risk
- Changes in emotion processing network activity, size of hippocampus
- Heterogeneous
- No simple link to a specific NT system
Next time…
- Schizophrenia
References
Audhya, Tapan, James B Adams, and Leah Johansen. 2012. “Correlation of Serotonin Levels in CSF, Platelets, Plasma, and Urine.” Biochimica et Biophysica Acta 1820 (10): 1496–1501. doi:10.1016/j.bbagen.2012.05.012.
Baldessarini, Ross J., Maurizio Pompili, and Leonardo Tondo. 2006. “Suicide in Bipolar Disorder: Risks and Management.” CNS Spectrums 11 (06): 465–71. doi:10.1017/S1092852900014681.
Berman, R M, A Cappiello, A Anand, D A Oren, G R Heninger, D S Charney, and J H Krystal. 2000. “Antidepressant Effects of Ketamine in Depressed Patients.” Biol. Psychiatry 47 (4): 351–54. https://www.ncbi.nlm.nih.gov/pubmed/10686270.
Burke, Heather M, Mary C Davis, Christian Otte, and David C Mohr. 2005. “Depression and Cortisol Responses to Psychological Stress: A Meta-Analysis.” Psychoneuroendocrinology 30 (9): 846–56. doi:10.1016/j.psyneuen.2005.02.010.
Cheng, Wei, Edmund T. Rolls, Jiang Qiu, Wei Liu, Yanqing Tang, Chu-Chung Huang, XinFa Wang, et al. 2016. “Medial Reward and Lateral Non-Reward Orbitofrontal Cortex Circuits Change in Opposite Directions in Depression.” Brain, October, aww255. doi:10.1093/brain/aww255.
Craddock, Nick, and Pamela Sklar. 2013. “Genetics of Bipolar Disorder.” The Lancet 381 (9878): 1654–62. doi:10.1016/S0140-6736(13)60855-7.
Dierckx, Bram, Willemijn T Heijnen, Walter W van den Broek, and Tom K Birkenhäger. 2012. “Efficacy of Electroconvulsive Therapy in Bipolar Versus Unipolar Major Depression: A Meta-Analysis.” Bipolar Disorders 14 (2): 146–50. doi:10.1111/j.1399-5618.2012.00997.x.
Fitzgerald, Paul B., Angela R. Laird, Jerome Maller, and Zafiris J. Daskalakis. 2008. “A Meta-Analytic Study of Changes in Brain Activation in Depression.” Human Brain Mapping 29 (6): 683–95. doi:10.1002/hbm.20426.
Geddes, John R, and David J Miklowitz. 2013. “Treatment of Bipolar Disorder.” The Lancet 381 (9878): 1672–82. doi:10.1016/S0140-6736(13)60857-0.
Goldstein-Piekarski, Andrea N., Mayuresh S. Korgaonkar, Erin Green, Trisha Suppes, Alan F. Schatzberg, Trevor Hastie, Charles B. Nemeroff, and Leanne M. Williams. 2016. “Human Amygdala Engagement Moderated by Early Life Stress Exposure Is a Biobehavioral Target for Predicting Recovery on Antidepressants.” Proceedings of the National Academy of Sciences 113 (42): 11955–60. doi:10.1073/pnas.1606671113.
Hallahan, Brian, John Newell, Jair C. Soares, Paolo Brambilla, Stephen M. Strakowski, David E. Fleck, Tuula Kieseppä, et al. 2011. “Structural Magnetic Resonance Imaging in Bipolar Disorder: An International Collaborative Mega-Analysis of Individual Adult Patient Data.” Biological Psychiatry, Bipolar Disorder: Genes and Brain Development, 69 (4): 326–35. doi:10.1016/j.biopsych.2010.08.029.
Hamilton, J Paul, Amit Etkin, Daniella J Furman, Maria G Lemus, Rebecca F Johnson, and Ian H Gotlib. 2012. “Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data.” AJP 169 (7). American Psychiatric Publishing: 693–703. doi:10.1176/appi.ajp.2012.11071105.
Lawrence, Natalia S, Andrew M Williams, Simon Surguladze, Vincent Giampietro, Michael J Brammer, Christopher Andrew, Sophia Frangou, Christine Ecker, and Mary L Phillips. 2004. “Subcortical and Ventral Prefrontal Cortical Neural Responses to Facial Expressions Distinguish Patients with Bipolar Disorder and Major Depression.” Biological Psychiatry 55 (6): 578–87. doi:10.1016/j.biopsych.2003.11.017.
Leung, Joseph, Caroline Selvage, Taryn Bosdet, Jennifer Branov, Annie Rosen-Heath, Carole Bishop, Sandra Sirrs, and Gabriella Horvath. 2018. “Salivary Serotonin Does Not Correlate with Central Serotonin Turnover in Adult Phenylketonuria (PKU) Patients.” Molecular Genetics and Metabolism Reports 15 (June): 100–105. doi:10.1016/j.ymgmr.2018.03.008.
Li, Nanxin, Boyoung Lee, Rong-Jian Liu, Mounira Banasr, Jason M Dwyer, Masaaki Iwata, Xiao-Yuan Li, George Aghajanian, and Ronald S Duman. 2010. “mTOR-dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists.” Science 329 (5994): 959–64. doi:10.1126/science.1190287.
Mahar, Ian, Francis Rodriguez Bambico, Naguib Mechawar, and José N. Nobrega. 2014. “Stress, Serotonin, and Hippocampal Neurogenesis in Relation to Depression and Antidepressant Effects.” Neuroscience & Biobehavioral Reviews 38 (January): 173–92. doi:10.1016/j.neubiorev.2013.11.009.
Malhi, Gin S., Michelle Tanious, Pritha Das, Carissa M. Coulston, and Michael Berk. 2013. “Potential Mechanisms of Action of Lithium in Bipolar Disorder.” CNS Drugs 27 (2): 135–53. doi:10.1007/s40263-013-0039-0.
Medici, Marco, Nese Direk, W Edward Visser, Tim I M Korevaar, Albert Hofman, Theo J Visser, Henning Tiemeier, and Robin P Peeters. 2014. “Thyroid Function Within the Normal Range and the Risk of Depression: A Population-Based Cohort Study.” J. Clin. Endocrinol. Metab. 99 (4): 1213–9. doi:10.1210/jc.2013-3589.
Palazidou, Eleni. 2012. “The Neurobiology of Depression.” British Medical Bulletin 101 (February): 127–45. doi:10.1093/bmb/lds004.
Ruhé, H G, N S Mason, and A H Schene. 2007. “Mood Is Indirectly Related to Serotonin, Norepinephrine and Dopamine Levels in Humans: A Meta-Analysis of Monoamine Depletion Studies.” Molecular Psychiatry 12 (4): 331–59. doi:10.1038/sj.mp.4001949.
Samuelsson, M., J. Jokinen, A.-L. Nordström, and P. Nordström. 2006. “CSF 5-HIAA, Suicide Intent and Hopelessness in the Prediction of Early Suicide in Male High-Risk Suicide Attempters.” Acta Psychiatrica Scandinavica 113 (1): 44–47. doi:10.1111/j.1600-0447.2005.00639.x.
Tiger, Mikael, Per Svenningsson, Magdalena Nord, Sandra Jabre, Christer Halldin, and Johan Lundberg. 2015. “No Correlation Between Serotonin and Its Metabolite 5-HIAA in the Cerebrospinal Fluid and [11c]AZ10419369 Binding Measured with PET in Healthy Volunteers,” February. Inst för klinisk neurovetenskap / Dept of Clinical Neuroscience. http://hdl.handle.net/10616/44513.
Videbech, Poul, and Barbara Ravnkilde. 2004a. “Hippocampal Volume and Depression: A Meta-Analysis of Mri Studies.” American Journal of Psychiatry 161 (11). Am Psychiatric Assoc: 1957–66. doi:10.1176/appi.ajp.161.11.1957.
———. 2004b. “Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies.” Am. J. Psychiatry 161 (11): 1957–66. doi:10.1176/appi.ajp.161.11.1957.
Zarate, Carlos A, Jr, Jaskaran B Singh, Paul J Carlson, Nancy E Brutsche, Rezvan Ameli, David A Luckenbaugh, Dennis S Charney, and Husseini K Manji. 2006. “A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression.” Arch. Gen. Psychiatry 63 (8): 856–64. doi:10.1001/archpsyc.63.8.856.