2022-02-15 08:16:48
Prelude (4:13)
Prelude (4:44)
Today’s Topics
- How neurons talk to one another
- Synaptic communication
- Neurotransmitters
How neurons talk to one another
In the beginning
- Soma receives input from dendrites
- Axon hillock sums/integrates
- If sum > threshold, AP “fires”
Illustration of summation
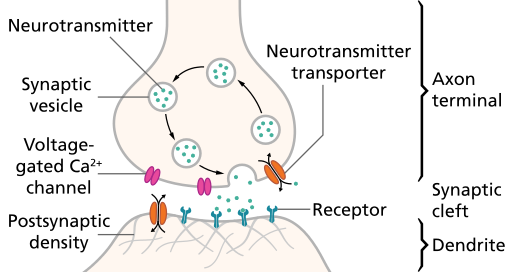
Steps in synaptic transmission
- Rapid change in voltage triggers neurotransmitter (NT) release
- Voltage-gated calcium Ca++ channels open
- Ca++ causes synaptic vesicles to bind with presynaptic membrane & merge with it
- NTs released via exocytosis

Steps in synaptic transmission
- NTs diffuse across synaptic cleft
- NTs bind with receptors on postsynaptic membrane
- Receptors respond
- NTs unbind, are inactivated
Synaptic transmission
Exocytosis
Why do NTs move from presynaptic terminal toward postsynaptic cell?
- Electrostatic force pulls them
- Force of diffusion
Why do NTs move from presynaptic terminal toward postsynaptic cell?
Electrostatic force pulls them- Force of diffusion
Relative sizes
- Neural membrane ~8 nm
- Synaptic vesicles ~40-60 or ~90-120 nm
- Synaptic cleft ~15-50 nm
- Cleft small relative to vesicles, so diffusion time short (< 0.5 ms)
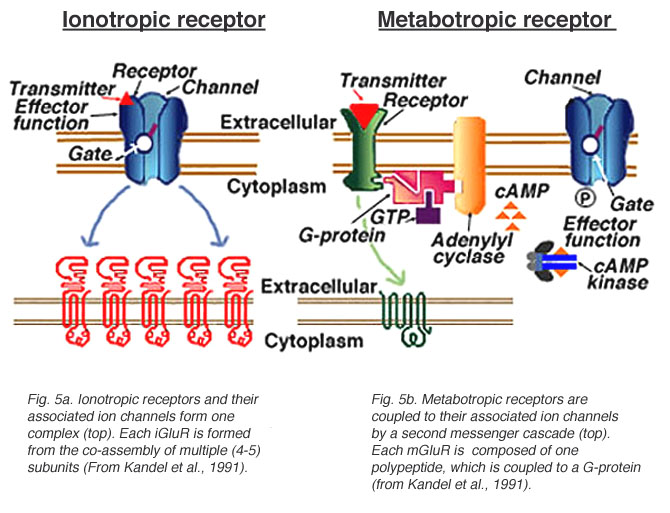
Postsynaptic receptor types
- Ionotropic (receptor + ion channel)
- Ligand-gated
- Open/close ion channel
- Ions flow in/out depending on membrane voltage and ion type
- Fast-responding (< 2 ms), but short-duration effects (< 100 ms)
Postsynaptic receptor types
- Metabotropic (receptor only, no attached ion channels
- Trigger G-proteins attached to receptor
- G-proteins activate 2nd messengers
- 2nd messengers bind to, open/close adjacent channels or change metabolism
- Slower, but longer-lasting effects
Receptor types
Receptors generate postsynaptic potentials (PSPs)
- Small voltage changes
- Amplitude scales with # of receptors activated
- Number of receptors activated ~ # of vesicles released
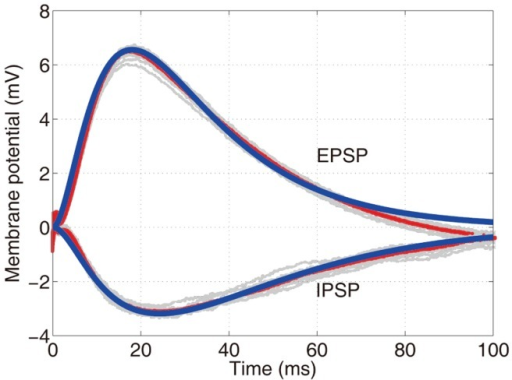
Postsynaptic potential types
- Excitatory PSPs (EPSPs)
- Depolarize neuron (make more +)
- Move membrane potential closer to threshold
- Inhibitory (IPSPs)
- Hyperpolarize neuron (make more -)
- Move membrane potential away from threshold
Mechanisms of NT inactivation
- Buffering
- e.g., glutamate into astrocytes (Anderson & Swanson, 2000)
- Reuptake via transporters
- molecules in membrane that move NTs inside
- e.g., serotonin via serotonin transporter (SERT)
- Enzymatic degradation
- e.g., Acetylcholinesterase (AChE) degrades acetylcholine (ACh)
Questions to ponder
- Why must NTs be inactivated?
Questions to ponder
- Why must NTs be inactivated?
- Keeps messages discrete, localized in time and space
What sort of PSP would opening a Na+ channel produce?
- Excitatory PSP, Na+ flows in
- Excitatory PSP, Na+ flows out
- Inhibitory PSP, Na+ flows in
- Inhibitory PSP, Na+ flows out
What sort of PSP would opening a Na+ channel produce?
- Excitatory PSP, Na+ flows in
- Excitatory PSP, Na+ flows out
- Inhibitory PSP, Na+ flows in
- Inhibitory PSP, Na+ flows out
What sort of PSP would opening a Cl- channel produce?
Remember [Cl-out]>>[Cl-in]; Assume resting potential ~60 mV
- Excitatory PSP, Cl- flows in
- Excitatory PSP, Cl- flows out
- Inhibitory PSP, Cl- flows in
- Inhibitory PSP, Cl- flows out
What sort of PSP would opening a Cl- channel produce?
Remember [Cl-out]>>[Cl-in]; Assume resting potential ~-60 mV
- Excitatory PSP, Cl- flows in
- Excitatory PSP, Cl- flows out
- Inhibitory PSP, Cl- flows in
- Inhibitory PSP, Cl- flows out
Types of synapses
Types of synapses
- Axodendritic (axon to dendrite)
- Axosomatic (axon to soma)
- Axoaxonic (axon to axon)
- Axosecretory (axon to bloodstream)
Synapses on
- dendrites
- usually excitatory
- cell bodies
- usually inhibitory
- axons
- usually modulatory (change p(fire))
Summary of chemical communication
Neurotransmitters
What are they?
- Chemicals produced by neurons
- Released by neurons
- Bound by neurons and other cells
- Send messages (have physiological effect on target cells)
- Inactivated after release
Neurotransmiters
| Family | Neurotansmitter |
|---|---|
| Amino acids | Glutamate (Glu) |
| Gamma aminobutyric acid (GABA) | |
| Glycine | |
| Aspartate |
Glutamate
- Primary excitatory NT in CNS (~ 1/2 all synapses)
- Role in learning (via NMDA receptor)
- Transporters on neurons and glia (astrocytes and oligodendrocytes)
- Linked to umami (savory) taste sensation, think monosodium glutamate (MSG)
- Dysregulation in schizophrenia (McCutcheon, Krystal, & Howes, 2020), mood disorders (Małgorzata, Paweł, Iwona, Brzostek, & Andrzej, 2020)
Glutamate
| Type | Receptor | Esp Permeable to |
|---|---|---|
| Ionotropic | AMPA | Na+, K+ |
| Kainate | ||
| NMDA | Ca++ | |
| Metabotropic | mGlu |
\(\gamma\)-aminobutyric Acid (GABA)
- Primary inhibitory NT in CNS
- Excitatory in developing CNS, [Cl-] in >> [Cl-] out
- Binding sites for benzodiazepines (e.g., Valium), barbiturates, ethanol, etc.
- Synthesized from glutamate
- Inactivated by transporters
| Type | Receptor | Esp Permeable to |
|---|---|---|
| Ionotropic | GABA-A | Cl- |
| Metabotropic | GABA-B | K+ |
GABA

“GABAA-receptor-protein-example” by Chemgirl131 at English Wikipedia - Transferred from
en.wikipedia to Commons by Sreejithk2000 using CommonsHelper.. Licensed under Public Domain via Commons.
Other amino acid NTs
- Glycine
- Spinal cord interneurons
- Also inhibitory
- Aspartate
- Like Glu, stimulates NMDA receptor
Acetylcholine (ACh)
- Primary NT of CNS output
- Somatic nervous system (neuromuscular junction)
- Autonomic nervous system
- Sympathetic branch: preganglionic neuron
- Parasympathetic branch: pre/postganglionic
- Inactivation by acetylcholinesterase (AChE)
ACh anatomy
Acetylcholine
| Type | Receptor | Esp Permeable to | Blocked by |
|---|---|---|---|
| Ionotropic | Nicotinic (nAChR) | Na+, K+ | e.g., Curare |
| Metabotropic | Muscarinic (mAChR) | K+ | e.g., Atropine |
Curare
Atropine
- aka, nightshade or belladonna
How to stop your prey
| Substance | Effect |
|---|---|
| Japanese pufferfish toxin | Blocks voltage-gated Na+ channels |
| Black widow spider venom | Accelerates presynaptic ACh release |
| Botulinum toxin (BoTox) | Prevents ACh vesicles from binding presynaptically |
| Sarin nerve gas | Impedes ACh breakdown by AChE |
| Pesticides | Impede AChE |
| Tetanus toxin | Blocks release of GABA, glycine |
Next time…
- More on NTs!
References
acapellascience. (2017, June). The molecular shape of you (ed sheeran parody) | a capella science. Youtube. Retrieved from https://www.youtube.com/watch?v=f8FAJXPBdOg
Anderson, C. M., & Swanson, R. A. (2000). Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia, 32(1), 1–14. https://doi.org/10.1002/1098-1136(200010)32:1<1::AID-GLIA10>3.0.CO;2-W
Byrne, D. (2018, March). David byrne - here (official audio). Youtube. Retrieved from https://www.youtube.com/watch?v=T5TdD3eZjnM
Hastoy, B., Clark, A., Rorsman, P., & Lang, J. (2017). Fusion pore in exocytosis: More than an exit gate? A \(\beta\)-cell perspective. Cell Calcium, 68, 45–61. https://doi.org/10.1016/j.ceca.2017.10.005
Haucke, V., Neher, E., & Sigrist, S. J. (2011). Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nature Reviews. Neuroscience, 12(3), 127–138. https://doi.org/10.1038/nrn2948
Małgorzata, P., Paweł, K., Iwona, M. L., Brzostek, T., & Andrzej, P. (2020). Glutamatergic dysregulation in mood disorders: Opportunities for the discovery of novel drug targets. Expert Opinion on Therapeutic Targets, 24(12), 1187–1209. https://doi.org/10.1080/14728222.2020.1836160
McCutcheon, R. A., Krystal, J. H., & Howes, O. D. (2020). Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry: Official Journal of the World Psychiatric Association, 19(1), 15–33. https://doi.org/10.1002/wps.20693
![[[@Hastoy2017-it]](https://doi.org/10.1016/j.ceca.2017.10.005)](https://ars.els-cdn.com/content/image/1-s2.0-S0143416017301495-fx1.jpg)
![[[@Hastoy2017-it]](https://doi.org/10.1016/j.ceca.2017.10.005)](https://ars.els-cdn.com/content/image/1-s2.0-S0143416017301495-gr1_lrg.jpg)
![[[@Haucke2011-ub]](http://dx.doi.org/10.1038/nrn2948)](https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fnrn2948/MediaObjects/41583_2011_Article_BFnrn2948_Fig1_HTML.jpg?as=webp)