2022-03-15 16:54:54
Prelude
Today’s Topics
- The neuroscience of psychiatric disorders
- Major affective (mood) disorders
- Major Depressive Disorder (depression)
- Bipolar Disorder
Serious Mental Illness among Adults in the Past Year
Neuroscience of psychiatric disorders
- Diagnosis via behavior & mood not specific “biomarker”
- Presume diseases of the mind are disorders of the brain
- System-wide effects; no single or simple cause
Neuroscience of psychiatric disorders
- Heritability
- proportion of variance in trait accounted for by genetic factors
- Higher for psychiatric disorders than non-psychiatric diseases
- Family member with mental illness highest known risk factor
Depression
Major Depressive Disorder
- Symptoms
- Unhappy mood, insomnia, lethargy, loss of pleasure, interest, energy
- Agitation
- Lasting for several weeks or more
Symptoms
Depression
- Experienced by ~7% Americans in any year
- Prevalence (up to ~20% lifetime)
- Females 2-3x males, higher 40+ years of age
- Heritability (large, 2.5 M Swedish population study)
- Females 0.49 (twins); 0.51 (non-twin relatives)
- Males 0.41 (twins); 0.36 (non-twin relatives)
- (Kendler, Ohlsson, Lichtenstein, Sundquist, & Sundquist, 2018)
Neurobiology of Major Depressive Disorder (MDD)
- Reduced sizes of brain regions
- Hypoactivity
- Pharmacological factors
- Synaptic dysfunction
MDD: Neurological factors
- Reduced hippocampal volumes
- (Videbech & Ravnkilde, 2004a) meta-analysis
(Videbech & Ravnkilde, 2004a)
Left Hippocampus
(Videbech & Ravnkilde, 2004b)
Right Hippocampus
MDD: Neurological factors
- Hypoactivity (Fitzgerald, Laird, Maller, & Daskalakis, 2008) in
- Frontal and temporal cortex
- Anterior cingulate
- Insula
- Cerebellum
(Fitzgerald et al., 2008)
Row (a) patients v. controls, (b) patients on SSRIs, (c) patients v. ctrls (happy stim), (d) patients v. controls (sad stim)
`
Neurological factors
- Hyperactivity (Hamilton et al., 2012)
- At baseline: in pulvinar nucleus of thalamus
- In response to negative stimuli: amygdala, insula, anterior cingulate
- Hypoactivity
- In response to negative stimuli: prefrontal cortex, striatum of basal ganglia
Baseline hyperactivity (Hamilton et al., 2012)
Hyper/hypo-activity specific to emotional valence (Hamilton et al., 2012)
Disrupted connectivity
- Resting state fMRI (rsFMRI) in \(n=421\) patients with major depressive disorder and \(n=488\) control subjects.
- Reduced connectivity between orbitofrontal cortex (OFC) and other areas of the brain
- Increased connectivity between lateral PFC and other brain areas
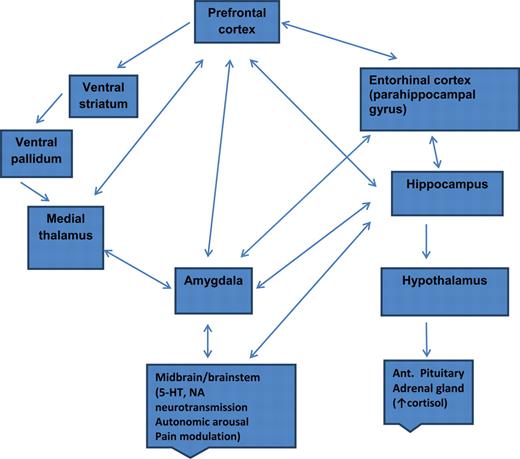
MDD: Network of areas implicated
Pharmacological factors
- Endocrine
- Thyroid dysfunction (Medici et al., 2014)
- Altered cortisol reactivity (Burke, Davis, Otte, & Mohr, 2005)
MDD: Pharmacological factors
- Monoamine hypothesis
- More: euphoria
- Less: depression
- Reserpine (antagonist for NE & 5-HT) can cause depression
- Low serotonin (5-HT) metabolite levels in CSF of suicidal depressives (Samuelsson, Jokinen, Nordström, & Nordström, 2006)
Measuring 5-HT
- CSF, platelets, plasma, urine, saliva
- CSF & platelets correlate highly (Audhya, Adams, & Johansen, 2012)
- But, salivary 5-HT does not correlate with mood symptoms (Leung et al., 2018)
MDD: Pharmacological factor summary
Treatments for depression
- Psychotherapy
- Often effective when combined with drug treatment
- Exercise
- Drugs
Drugs
- Monoamine oxidase (MAO) inhibitors
- MAO destroys excess monoamines in terminal buttons & glia
- MAO-I’s boost monoamine levels
- Tricyclics
- Inhibit NE, 5-HT reuptake
- Upregulate monoamine levels, but non-selective => side effects
Drugs
- Selective Serotonin Reuptake Inhibitors (SSRIs)
- Fluoxetine (Prozac, Paxil, Zoloft)
- Prolong duration of 5-HT in synaptic cleft
- Also increase brain steroid production
- Selective Serotonin Norepinephrine Reuptake Inhibitors (SNRIs)
Cymbalta (SNRI)
How well do the drugs work?
- STAR*D trial
- On SSRI for 12-14 weeks. ~1/3 achieved remission; 10-15% showed symptom reduction.
- If SSRI didn’t work, could switch drugs. ~25% became symptom free.
- 16% of participants dropped out due to tolerability issues
- 6-7 weeks to show response
Who benefits from drug therapy?
- Depends on
- Early life stress (ELS)
- Brain (amygdala) response to emotional faces
- (Goldstein-Piekarski et al., 2016)
- Low ELS + low amyg reactivity > responding
- High ELS + high amyg reactivity > responding
Monoamine hypothesis of depression
- Disrupted (lowered) levels of monoamines (especially NE & 5-HT) result in depression
Problems with monoamine hypothesis
- Too simplistic
- NE, 5-HT interact
- Drugs fast acting (min), but improvement slow (weeks)
“No correlation between serotonin and its metabolite 5-HIAA in the cerebrospinal fluid and [11C]AZ10419369 binding measured with PET in healthy volunteers.” (Tiger et al., 2015)
“…we performed the first meta-analysis of the mood effects in [acute tryptophan depletion] ATD and [alpha-methyl-para-tyrosine] APTD studies. The depletion of monoamine systems (both 5-HT and NE/DA) does not decrease mood in healthy controls. However, in healthy controls with a family history of MDD the results suggest that mood is slightly decreased…by [monoamine depletion]…”
What do drugs do, then?
- Alter receptor sensitivity?
- 5-HT presynaptic autoreceptors compensate
- Postsynaptic upregulation of NE/5-HT effects
What do drugs do, then?
- Stimulate neurogenesis?
- Link to neurotrophin, brain-derived nerve growth factor (BDNF)
- BDNF boosts neurogenesis
- SSRIs stimulate growth of new neurons in hippocampus
Neurogenesis hypothesis, (Mahar et al., 2014)
- Chronic stress causes neural loss in hipp
- Chronic stress downregulates 5-HT sensitivity
- Depression ~ chronic stress
- Anti-depressants upregulate neurogenesis via 5-HT modulation
Ketamine
- Selective antagonist of the NMDA receptor, an ionotropic glutamate receptor
- Relieves depressive symptoms relatively quickly (Berman et al., 2000) and (Zarate et al., 2006)
- Boosts synaptic spine formation (Li et al., 2010) and reverses effects of induced stress
Electroconvulsive Therapy (ECT)
- Last line of treatment for drug-resistant depression
- Electric current delivered to the brain causes 30-60s seizure.
- ECT usually done in a hospital’s operating or recovery room under general anesthesia
- Once every 2 - 5 days for a total of 6 - 12 sessions.
Electroconvulsive Therapy (ECT)
- Remission rates of up to 50.9% (Dierckx, Heijnen, Broek, & Birkenhäger, 2012)
- Seems to work via
- Anticonvulsant (block Na+ channel or enhance GABA function) effects
- Neurotrophic (stimulates neurogenesis) effects
ECT more effective than Ketamine?
![Fig 3: [[@Ekstrand2021-cq]](http://dx.doi.org/10.1093/ijnp/pyab088)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijnp/PAP/10.1093_ijnp_pyab088/2/pyab088_fig3.jpeg?Expires=1650372654&Signature=vHS8iH7DVvu35s~WV0UC1x~~B--49hY2X4uab3DUI9qAT0jA0F7bbbE2Dlcvk5BgBoC6AfqTfgXUr67koz1hnhAe5DKgtMJoxOUl9v3ZsHUqvJVxfYOPFkc1HvqZf231oynSVLuXEtuUyjREX-4V1cSVBhM1iJpmkeMrPnfHwehq1Q3FJkaU5E6dtJyAz8rLqeDZEXo4C954Q0nANq6-lw3LlbEiUzXOsDnZPLY~8XW7WuMZAYt5CRxOl7ITUXAnzqTp~7k6HXbPJBTQcriTu2iw1O-LQRdthW8zuVSNOXZrSiaqe8eNX4fJ92q30je2mSNJG2b3MHQ4FO6phmjrmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Fig 3: (Ekstrand et al., 2021)
The promise of deep brain stimuluation
Depression’s impact
- Widespread brain dysfunction
- Prefrontal cortex, amygdala, HPA axis, circadian rhythms
- Genetic + environmental factors
- Disturbance in 5-HT, NE systems, cortisol
- Metabolic pathways (Pu et al., 2020)
- Many sufferers do not respond to available treatments
Points on depression
- Drug treatments affect neuromodulator NT systems, but
- Can’t effectively measure NT levels
- Neuromodulators interact, so many side-effects
- ‘Monoamine hypothesis’ of depression is at-best incomplete
- ‘Talk’ therapies can change behavior/mood by creating new/strengthened circuits
- Emerging therapies (ketamine, deep brain stimulation) show promise, but…
“Leading biological hypotheses propose that biological changes may underlie major depressive disorder onset and relapse/recurrence. Here, we investigate if there is prospective evidence for biomarkers derived from leading theories. We focus on neuroimaging, gastrointestinal factors, immunology, neurotrophic factors, neurotransmitters, hormones, and oxidative stress….Our search resulted in 67,464 articles”
“…Only cortisol (N=19, OR=1.294, p=0.024) showed a predictive effect on onset/relapse/recurrence of MDD, but not on time until MDD onset/relapse/recurrence.” “However, this effect disappeared when studies including participants with a baseline clinical diagnosis were removed from the analyses…”
“…there is a lack of evidence for leading biological theories for onset and maintenance of depression. Only cortisol was identified as potential predictor for MDD, but results are influenced by the disease state. High-quality (prospective) studies on MDD are needed to disentangle the etiology and maintenance of MDD.”
Bipolar disorder
Bipolar disorder
- Formerly “manic depression” or “manic depressive disorder”
- Alternating mood states
- Mania or hypomania (milder form)
- Depression
- Cycles 3-6 mos in length, but
- Rapid cycling (weeks or days)
- Suicide risk 20-60x normal population, (Baldessarini, Pompili, & Tondo, 2006)
Symptoms
Prevalence, subtypes
- 1-3% lifetime prevalence, subthreshold affects another ~2% (Merikangas et al., 2007)
- Subtypes
- Bipolar I: manic episodes, possible depressive ones
- Bipolar II: no manic episodes but hypomania (disinhibition, irritability/agitation) + depression
Related symptoms
- Psychosis (hallucinations or delusions)
- Anxiety, attention-deficit hyperactivity disorder (ADHD)
- Substance abuse
Genetics
- Overlap between bipolar disorder and schizophrenia
- Genes for voltage-gated Ca++ channels
- Regulate NT, hormone release
- Gene expression, cell metabolism
- (Craddock & Sklar, 2013)
Brain responses to emotional faces ≠ depression
(Lawrence et al., 2004)
(Lawrence et al., 2004)
Amygdala, hippocampus volume reduced; ventricles larger
(Hallahan et al., 2011)
Drug treatments
- Anti-depressants not especially effective (Sidor & MacQueen, 2012)
- Mood stabilizers
- Lithium (Li)
- Valproate (Depakote)
- Anticonvulsants
- Usually to treat epilepsy
- GABA agonists
- e.g. lamotrigine (Lamictal)
- Atypical antipsychotics
Lithium “discovered” accidentally
- John Cade discovered in 1948
- Injections of manic patients’ urine with a lithium compound (chemical stabilizer) into guinea pig test animals
- Had calming effect
- Earliest effective medications for treating mental illness
Effects of lithium
- Reduces mania, minimal effects on depressive states
- Preserves PFC, hipp, amyg volumes
- Has other ‘neuroprotective’ effects (Machado-Vieira, Manji, & Zarate, 2009)
Effects of lithium
- downregulates DA, glutamate; upregulates GABA
- modulates 5-HT, NE
- levels can be tested/monitored via blood test
- (Malhi, Tanious, Das, Coulston, & Berk, 2013)
Other treatment options
- Psychotherapy
- Electroconvulsive Therapy (ECT)
- Sleep medications
Prospects
- STEP-BD cohort (\(n=1,469\))
- 58% achieved recovery
- 49% (of recovered) had recurrences within 2 years
- Residual depressive symptoms can persist
- (Geddes & Miklowitz, 2013)
An Unquiet Mind
BP summed-up
- Changes in mood, but ≠ depression
- Genetic + environmental risk
- Changes in emotion processing network activity, size of hippocampus
- Heterogeneous
- No simple link to a specific NT system
Next time…
- Schizophrenia
References
Audhya, T., Adams, J. B., & Johansen, L. (2012). Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochimica Et Biophysica Acta, 1820(10), 1496–1501. https://doi.org/10.1016/j.bbagen.2012.05.012
Baldessarini, R. J., Pompili, M., & Tondo, L. (2006). Suicide in Bipolar Disorder: Risks and Management. CNS Spectrums, 11(06), 465–471. https://doi.org/10.1017/S1092852900014681
Berman, R. M., Cappiello, A., Anand, A., Oren, D. A., Heninger, G. R., Charney, D. S., & Krystal, J. H. (2000). Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry, 47(4), 351–354. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10686270
Burke, H. M., Davis, M. C., Otte, C., & Mohr, D. C. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. https://doi.org/10.1016/j.psyneuen.2005.02.010
Cheng, W., Rolls, E. T., Qiu, J., Liu, W., Tang, Y., Huang, C.-C., … Feng, J. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain, aww255. https://doi.org/10.1093/brain/aww255
Craddock, N., & Sklar, P. (2013). Genetics of bipolar disorder. The Lancet, 381(9878), 1654–1662. https://doi.org/10.1016/S0140-6736(13)60855-7
Dierckx, B., Heijnen, W. T., Broek, W. W. van den, & Birkenhäger, T. K. (2012). Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: A meta-analysis. Bipolar Disorders, 14(2), 146–150. https://doi.org/10.1111/j.1399-5618.2012.00997.x
Ekstrand, J., Fattah, C., Persson, M., Cheng, T., Nordanskog, P., Åkeson, J., … Movahed Rad, P. (2021). Racemic ketamine as an alternative to electroconvulsive therapy for unipolar depression: A randomized, Open-Label, Non-Inferiority trial ( KetECT). The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum. https://doi.org/10.1093/ijnp/pyab088
Fitzgerald, P. B., Laird, A. R., Maller, J., & Daskalakis, Z. J. (2008). A meta-analytic study of changes in brain activation in depression. Human Brain Mapping, 29(6), 683–695. https://doi.org/10.1002/hbm.20426
Geddes, J. R., & Miklowitz, D. J. (2013). Treatment of bipolar disorder. The Lancet, 381(9878), 1672–1682. https://doi.org/10.1016/S0140-6736(13)60857-0
Goldstein-Piekarski, A. N., Korgaonkar, M. S., Green, E., Suppes, T., Schatzberg, A. F., Hastie, T., … Williams, L. M. (2016). Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proceedings of the National Academy of Sciences, 113(42), 11955–11960. https://doi.org/10.1073/pnas.1606671113
Hallahan, B., Newell, J., Soares, J. C., Brambilla, P., Strakowski, S. M., Fleck, D. E., … McDonald, C. (2011). Structural Magnetic Resonance Imaging in Bipolar Disorder: An International Collaborative Mega- Analysis of Individual Adult Patient Data. Biological Psychiatry, 69(4), 326–335. https://doi.org/10.1016/j.biopsych.2010.08.029
Hamilton, J. P., Etkin, A., Furman, D. J., Lemus, M. G., Johnson, R. F., & Gotlib, I. H. (2012). Functional neuroimaging of major depressive disorder: A Meta-Analysis and new integration of baseline activation and neural response data. AJP, 169(7), 693–703. https://doi.org/10.1176/appi.ajp.2012.11071105
Kendler, K. S., Ohlsson, H., Lichtenstein, P., Sundquist, J., & Sundquist, K. (2018). The genetic epidemiology of treated major depression in sweden. The American Journal of Psychiatry, 175(11), 1137–1144. https://doi.org/10.1176/appi.ajp.2018.17111251
Kennis, M., Gerritsen, L., Dalen, M. van, Williams, A., Cuijpers, P., & Bockting, C. (2020). Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Molecular Psychiatry, 25(2), 321–338. https://doi.org/10.1038/s41380-019-0585-z
Lawrence, N. S., Williams, A. M., Surguladze, S., Giampietro, V., Brammer, M. J., Andrew, C., … Phillips, M. L. (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry, 55(6), 578–587. https://doi.org/10.1016/j.biopsych.2003.11.017
Leung, J., Selvage, C., Bosdet, T., Branov, J., Rosen-Heath, A., Bishop, C., … Horvath, G. (2018). Salivary serotonin does not correlate with central serotonin turnover in adult phenylketonuria ( PKU) patients. Molecular Genetics and Metabolism Reports, 15, 100–105. https://doi.org/10.1016/j.ymgmr.2018.03.008
Li, N., Lee, B., Liu, R.-J., Banasr, M., Dwyer, J. M., Iwata, M., … Duman, R. S. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329(5994), 959–964. https://doi.org/10.1126/science.1190287
Machado-Vieira, R., Manji, H. K., & Zarate, C. A., Jr. (2009). The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disorders, 11 Suppl 2, 92–109. https://doi.org/10.1111/j.1399-5618.2009.00714.x
Mahar, I., Bambico, F. R., Mechawar, N., & Nobrega, J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neuroscience & Biobehavioral Reviews, 38, 173–192. https://doi.org/10.1016/j.neubiorev.2013.11.009
Malhi, G. S., Tanious, M., Das, P., Coulston, C. M., & Berk, M. (2013). Potential Mechanisms of Action of Lithium in Bipolar Disorder. CNS Drugs, 27(2), 135–153. https://doi.org/10.1007/s40263-013-0039-0
Medici, M., Direk, N., Visser, W. E., Korevaar, T. I. M., Hofman, A., Visser, T. J., … Peeters, R. P. (2014). Thyroid function within the normal range and the risk of depression: A population-based cohort study. J. Clin. Endocrinol. Metab., 99(4), 1213–1219. https://doi.org/10.1210/jc.2013-3589
Merikangas, K. R., Akiskal, H. S., Angst, J., Greenberg, P. E., Hirschfeld, R. M. A., Petukhova, M., & Kessler, R. C. (2007). Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Archives of General Psychiatry, 64(5), 543–552. https://doi.org/10.1001/archpsyc.64.5.543
Palazidou, E. (2012). The neurobiology of depression. British Medical Bulletin, 101, 127–145. https://doi.org/10.1093/bmb/lds004
Pu, J., Liu, Y., Zhang, H., Tian, L., Gui, S., Yu, Y., … Xie, P. (2020). An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Molecular Psychiatry. https://doi.org/10.1038/s41380-020-0645-4
Ruhé, H. G., Mason, N. S., & Schene, A. H. (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Molecular Psychiatry, 12(4), 331–359. https://doi.org/10.1038/sj.mp.4001949
Samuelsson, M., Jokinen, J., Nordström, A.-L., & Nordström, P. (2006). CSF 5- HIAA, suicide intent and hopelessness in the prediction of early suicide in male high-risk suicide attempters. Acta Psychiatrica Scandinavica, 113(1), 44–47. https://doi.org/10.1111/j.1600-0447.2005.00639.x
Sidor, M. M., & MacQueen, G. M. (2012). An update on antidepressant use in bipolar depression. Current Psychiatry Reports, 14(6), 696–704. https://doi.org/10.1007/s11920-012-0323-6
Tiger, M., Svenningsson, P., Nord, M., Jabre, S., Halldin, C., & Lundberg, J. (2015). No correlation between serotonin and its metabolite 5-HIAA in the cerebrospinal fluid and [ 11C]AZ10419369 binding measured with PET in healthy volunteers. Retrieved from http://hdl.handle.net/10616/44513
Videbech, P., & Ravnkilde, B. (2004a). Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry, 161(11), 1957–1966. https://doi.org/10.1176/appi.ajp.161.11.1957
Videbech, P., & Ravnkilde, B. (2004b). Hippocampal volume and depression: A meta-analysis of MRI studies. American Journal of Psychiatry, 161(11), 1957–1966. https://doi.org/10.1176/appi.ajp.161.11.1957
Zarate, C. A., Jr, Singh, J. B., Carlson, P. J., Brutsche, N. E., Ameli, R., Luckenbaugh, D. A., … Manji, H. K. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry, 63(8), 856–864. https://doi.org/10.1001/archpsyc.63.8.856



![[[@goldstein-piekarski_human_2016]](http://doi.org/10.1073/pnas.1606671113)](https://www.pnas.org/cms/10.1073/pnas.1606671113/asset/86493b5c-f70f-49d9-87e6-2a713d7021a3/assets/graphic/pnas.1606671113fig02.jpeg)