knitr::include_graphics("http://www.pitt.edu/~super1/lecture/lec14361/img024.GIF")Learning & Memory
PSY 511.003
Big picture questions
Memory capacity of the human brain?
- 1e12 neurons
- 1e3 synapses/neuron
- 1e15 synapses or 1.25e14 bytes
- 1e9 gigabyte, 1e12 terabyte, 1e15 petabyte
http://www.scientificamerican.com/article.cfm?id=what-is-the-memory-capacity
What is learning?
https://theelearningcoach.com/learning/10-definitions-learning/
Learning is the process of acquiring new understanding, knowledge, behaviors, skills, values, attitudes, and preferences. https://en.wikipedia.org/wiki/Learning
- Non-associative
- \(A(t+1) = f(A(t))\)
- Habituation (\(\dot f < 0\)), sensitization (\(\dot f > 0\))
- Associative
- \(A \rightarrow B\)
- Classical & operant/instrumental conditioning
- Sequence, observational, episodic, semantic…learning
Learning styles?
- Are myths (Pashler, McDaniel, Rohrer, & Bjork, 2008)?
Although the literature on learning styles is enormous, very few studies have even used an experimental methodology capable of testing the validity of learning styles applied to education. Moreover, of those that did use an appropriate method, several found results that flatly contradict the popular meshing hypothesis. We conclude therefore, that at present, there is no adequate evidence base to justify incorporating learning-styles assessments into general educational practice.
Neural networks
Network learning rules
- How/when change connections between nodes
- Types
- Unsupervised (i.e., covariance/correlation)
- Supervised (change based on ‘error’)
- Reinforcement (learn now)
Deep learning vs. Machine Learning
- (“Deep learning vs machine learning,” 2022; “Deep learning vs. Machine learning - azure machine learning,” n.d.)
What is memory?
- A: Information encoding, storage, retrieval
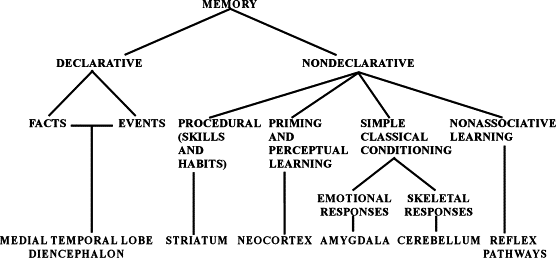
Are there different types of (human) memory?
- Facts/events/places/feelings vs. skills
- Short vs. long-term
- Working memory ~ short-term maintenance for guiding action
- Explicit (declarative: semantic vs. episodic) vs. implicit (procedural)
- Retrospective (from the past) vs. prospective (to be remembered)
- Recognition (judgment of familiarity or novelty) vs. recall
How do computers and brains compare?
| Computers | Brains |
|---|---|
| Computers have separate memory and processing stores | Brains store info everywhere, but there are specialized regions |
| Computer memory has specific addresses | Brains store in distributed networks |
| Computer memory is (usually) non-volatile | Memories in brains naturally fade |
Computer memory stores all types of information–images, sounds, text, data–as binary sequences, e.g., 01101110 |
Human memory stores all types of information in patterns of synaptic connections and ??? |
| Computers render these sequences differently based on information about the type of data stored | Brains retrieve or recall different forms of information based on ??? |
| Digital computers were inspired by mathematical models of neurons | Neurons can be simulated by mathematical models implemented in computers |

Biological bases…
In single cells
- Learning in single cells controversial (Gershman, Balbi, Gallistel, & Gunawardena, 2021)
- Beatrice Gelber

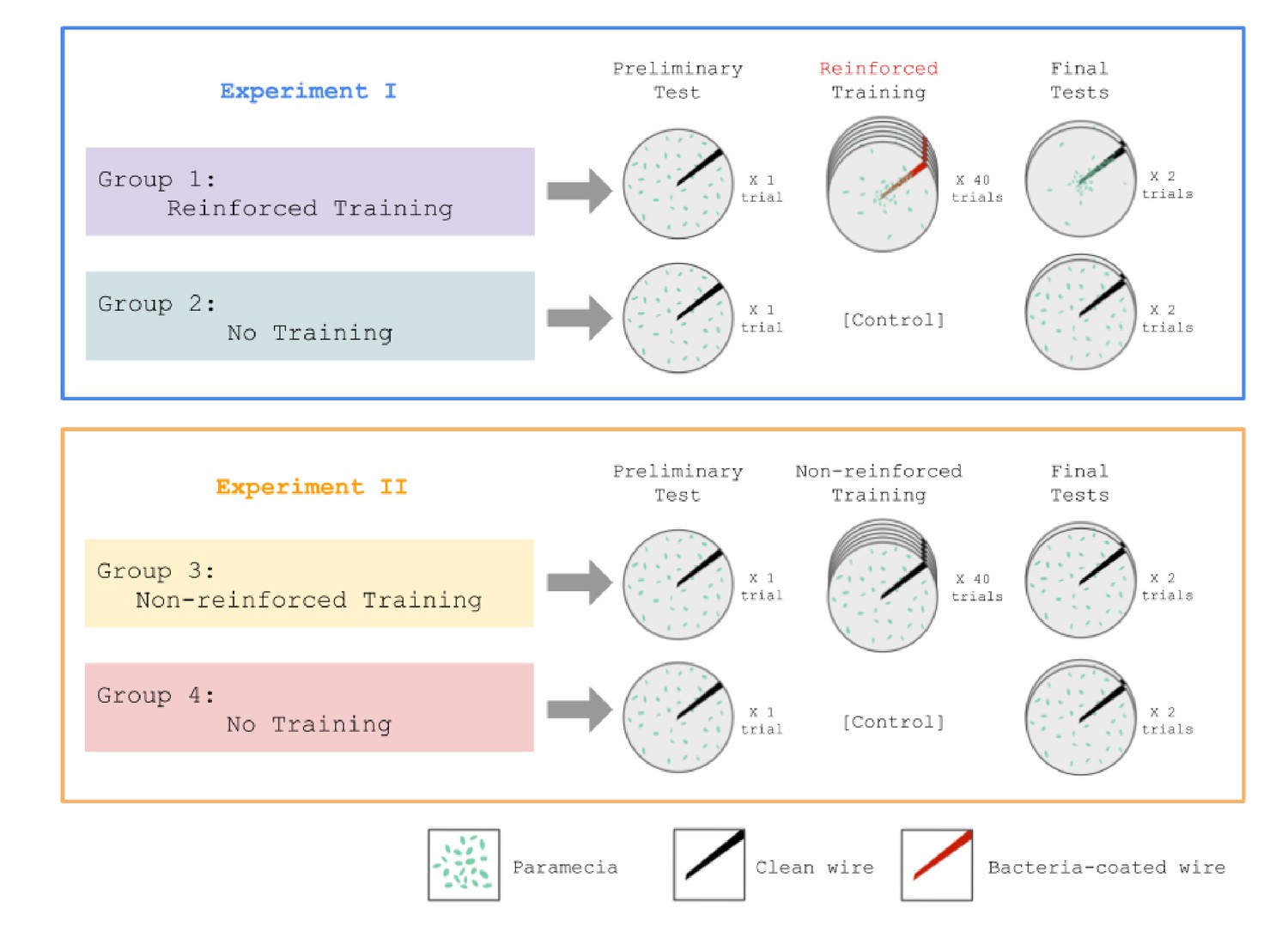
This paper presents a new approach to behavioral problems which might be called molecular biopsychology… Simply stated, it is hypothesized that the memory engram must be coded in macromolecules… As the geneticist studies the inherited characteristics of an organism the psychologist studies the modification of this inherited matrix by interaction with the environment. Possibly the biochemical and cellular physiological processes which encode new responses are continuous throughout the phyla (as genetic codes are) and therefore would be reasonably similar for a protozoan and a mammal. (Beatrice Gelber, 1962)
Taking stock, we believe that Gelber’s experiments, though not without their limitations, convincingly demonstrated Pavlovian conditioning in Paramecia. Sadly, her critics seem to have won in the long term. Most reviews of the literature, if they mention Gelber’s work at all, quickly dismiss it…(Gershman et al., 2021)
If single cells can learn then they must be using a non-synaptic form of memory storage. The idea that intracellular molecules store memories has a long history, mainly in the study of multicellular organisms. (Gershman et al., 2021)
In nervous systems
- Changes in patterns of neural activity
- Changes in connectivity
- New synapses
- Altered synapses (strengthened or weakened)
Donald Hebb’s Insight
“When an axon of cell A is near enough to excite cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficacy, as on of the cells firing B, is increased.”
(Hebb, 1949, p. 62)
“Neurons that fire together wire together.”
(Lowell & Singer, 1992, p. 211)
Long-term potentiation (LTP)
- An instance of Hebbian learning
“a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons.[2] The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.” https://en.wikipedia.org/wiki/Long-term_potentiation
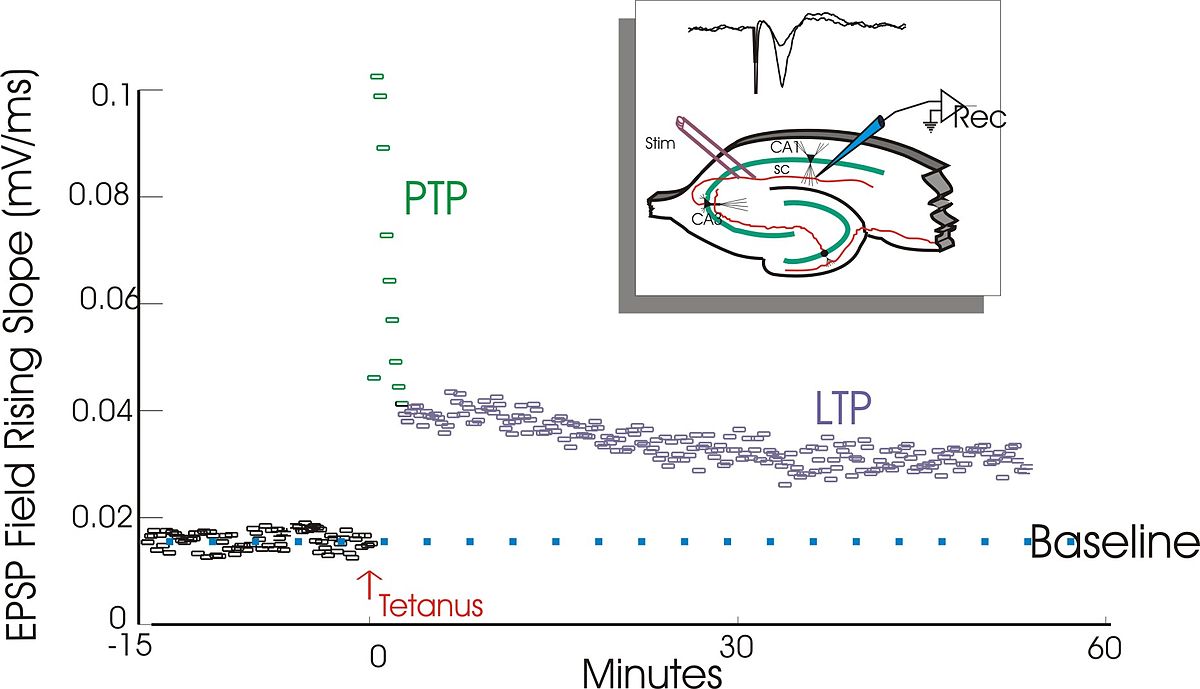
LTP discovery (Bliss & Lømo, 1973)
- Granule cell neurons in hippocampus dentate gyrus (DG)
- \(\theta\) band (10–20 Hz) stim for 10–15 sec, or 100 Hz stim for 3–4 sec
- shortened response latency, increased EPSP, increased population response over minutes or hours
Mechanisms of LTP plasticity
- number of synaptic receptors
- quantity of NT released
- effectiveness of postsynaptic response
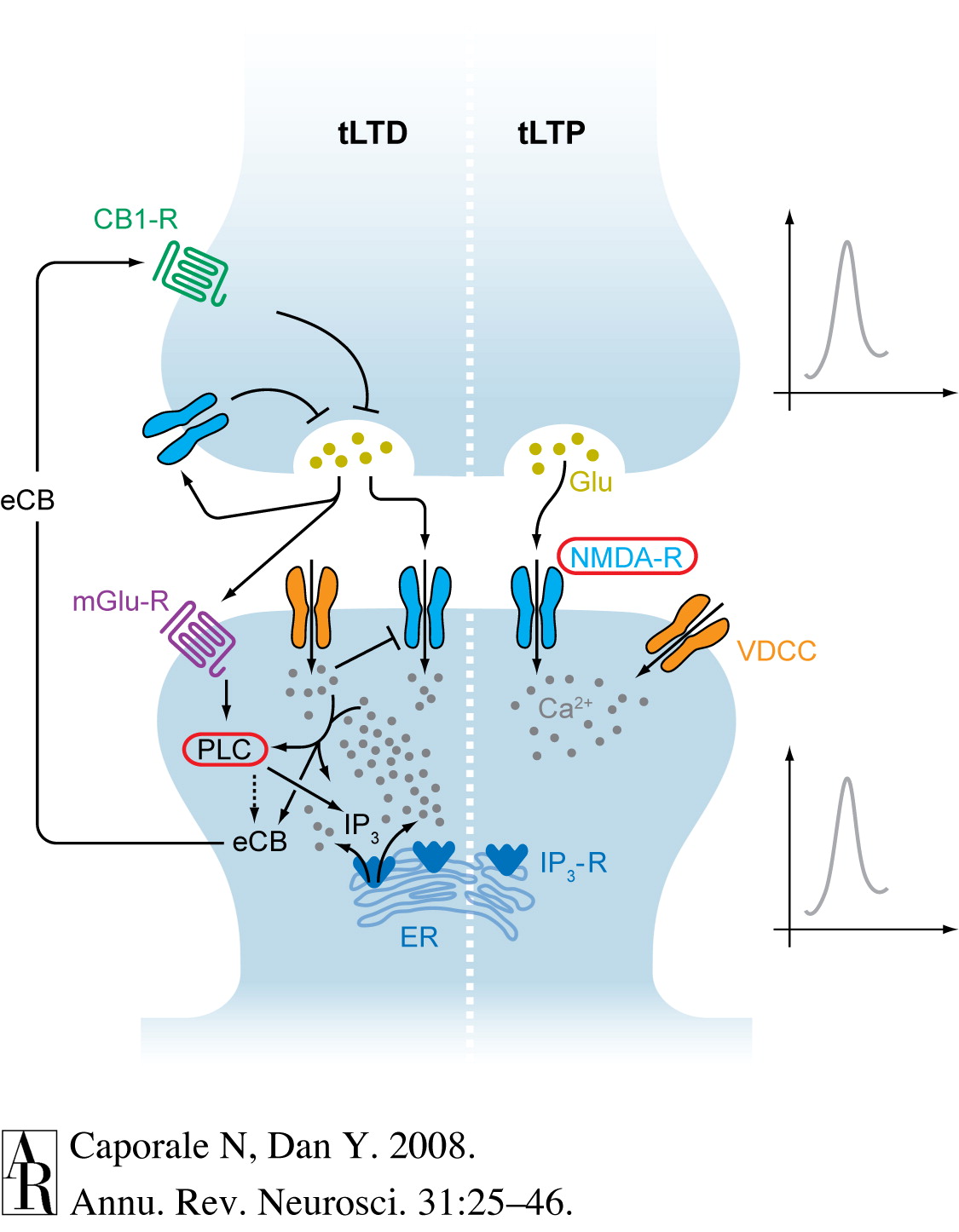
Pathways to plasticity
- Glu release activates
- ionotropic AMPA Glu receptors
- metabotropic Glu receptors
- N-methyl-D-aspartate (NMDA) Glu receptors
- \(Ca^{++}\) entry via NMDA receptor activates protein kinases (CaMKII and PKAII)
- Early LTP (up to few hours)
- protein kinases phosphorylate (add \(P\) group to) postsynaptic AMPA receptors
- Increase current flow through AMPA (Glu) receptors
- Late LTP
- depends on protein synthesis to generate new AMPA receptor
- insertion of new AMPA receptors into postsynaptic membrane
- Retrograde signal generator influences presynaptic response


‘Hebbian’ learning via NMDA receptor
- N-methyl-D-aspartate receptor (NMDA-R)
- Name derived from agonist molecule that selectively binds to the receptor
- ‘Coincidence’ detector
- Sending cell has released NT
- Receiving cell is/has been recently active
- Chemically-gated AND
- Ligand- (glutamate/aspartate + glycine) gated
- Sending cell active
- Voltage-gated
- \(Zn^{++}\) or \(Mg^{++}\) ion ‘plug’ removed under depolarization
- \(Na^+\) & \(Ca^{++}\) influx; \(K^+\) outflux
- Receiving cell responds
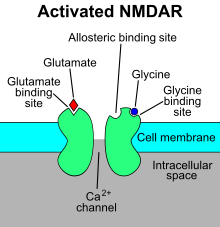
- NMDA receptor function may vary by location on neuron
- Long-term potentiation (LTP)
- Synaptic NMDA receptors
- Long-term depression (LTD)
- Extrasynaptic NMDA receptors
- Lowered level of synaptic receptor activation
- Long-term potentiation (LTP)
NMDA-R clinical significance
- Memantine (Alzheimer’s Disease treatment)
- NMDA-R antagonist
- Controls over-activation and \(Ca^{++}\) excitotoxicity?
- NMDA-R implicated in effects of phencylidine (PCP)
- Link to Glu hypothesis of schizophrenia?
- Ketamine is NMDA receptor antagonist
- anesthesia, sedation pain relief
- possible short-term relief for depression
- Linked to analgesic effects of nitrous oxide (laughing gas; \(NO\))
- Alcohol (ethanol) inhibits (Ron & Wang, 2011)
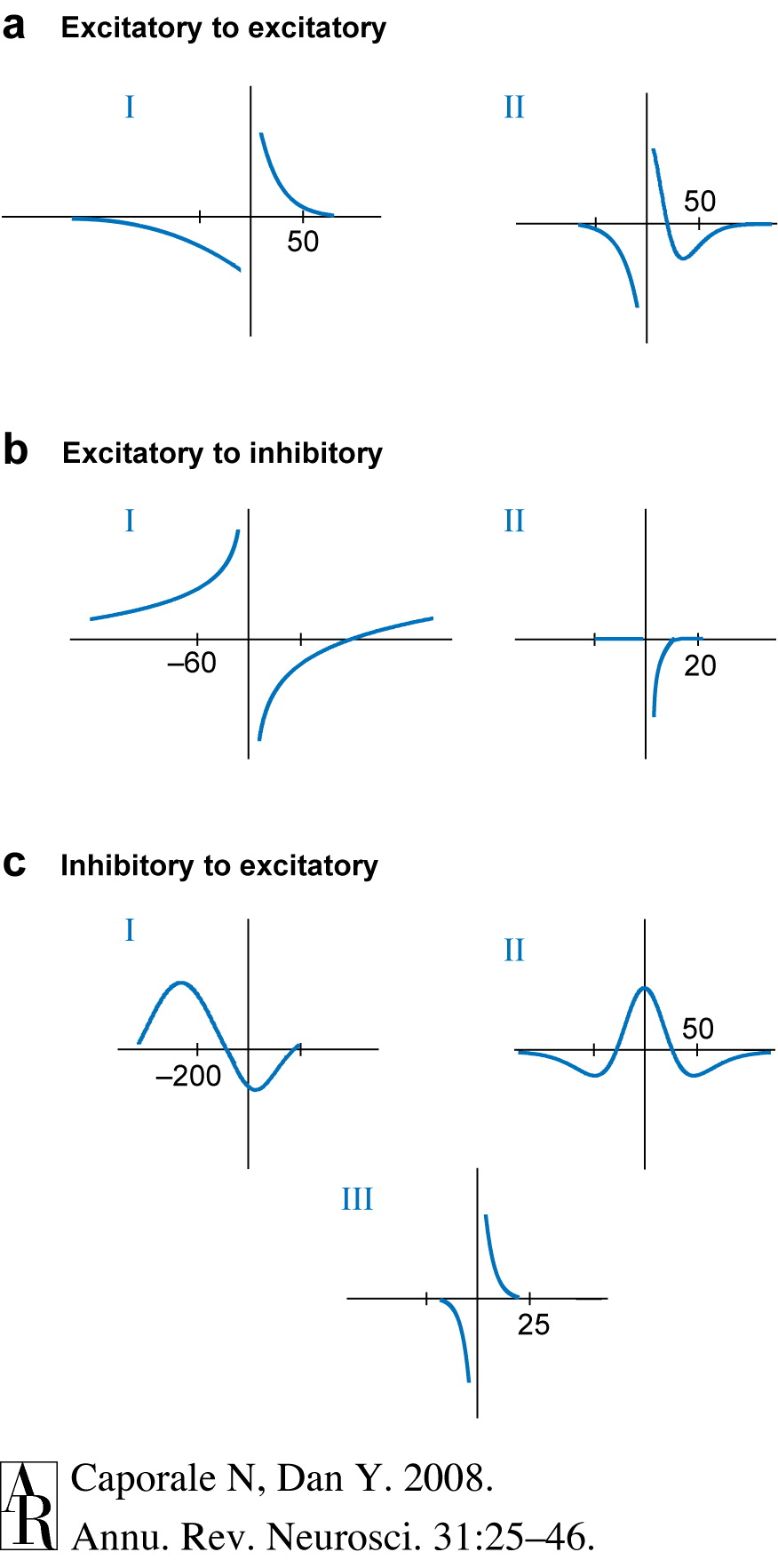
Spike-timing-dependent plasticity
How to learn/remember “causal chains”?
- e.g., lightning THEN thunder
- unusual food THEN indigestion
- A before B: strengthen \(A \rightarrow B\)
- A after B: \(A \rightarrow B\)
- Neural Plasticity
- Lasting changes in neural firing, connectivity
- NMDA receptor a molecular mechanism for implementing LTP and spike-timing-dependent plasticity
Memory systems
Lashley’s search for the ‘engram’
- Is memory ‘localized’ like sensory and motor functions? (Karl S. Lashley, 1944)
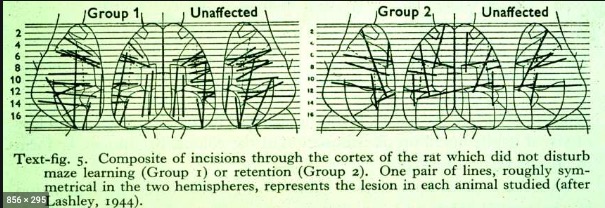
““the area subdivisions are in large part anatomically meaningless and misleading as to the presumptive functional divisions of the cortex””
- Not in the rat cerebral cortex
Modern views
- Cerebral cortex less central to “engram-like” memory than other areas
Hippocampus
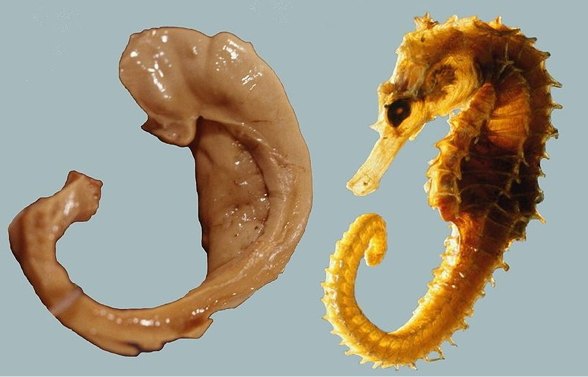
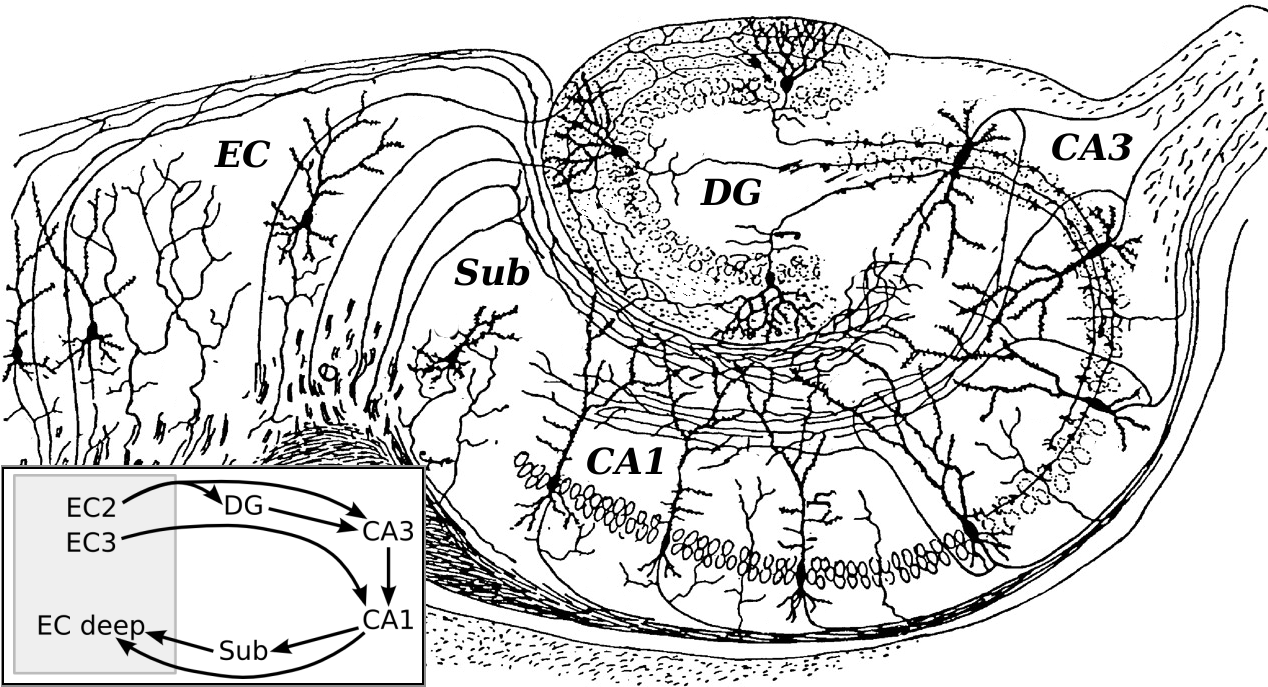
- Dense in NMDA receptors
- Central “hub” in network?
- No, based on anatomical or functional connectivity
- But yes, when modeling “information flow” (Mišić, Goñi, Betzel, Sporns, & McIntosh, 2014)
- Formation, storage, consolidation of long-term episodic or declarative memories
- Stores info for later transfer to cortex
- “Engrams” form in hipp & PFC simultaneously, fade in PFC over time (Kitamura et al., 2017)
- Spatial navigation
- London taxi drivers (a high memory demand profession) have specialized hippocampi (Maguire et al., 2000)
- Not due to self-motion, driving experience, or stress per se
Gray matter volume differences in the hippocampus relative to controls have been reported to accompany this expertise. While these gray matter differences could result from using and updating spatial representations, they might instead be influenced by factors such as self-motion, driving experience, and stress. We examined the contribution of these factors by comparing London taxi drivers with London bus drivers, who were matched for driving experience and levels of stress, but differed in that they follow a constrained set of routes. We found that compared with bus drivers, taxi drivers had greater gray matter volume in mid-posterior hippocampi and less volume in anterior hippocampi. Furthermore, years of navigation experience correlated with hippocampal gray matter volume only in taxi drivers, with right posterior gray matter volume increasing and anterior volume decreasing with more navigation experience. This suggests that spatial knowledge, and not stress, driving, or self-motion, is associated with the pattern of hippocampal gray matter volume in taxi drivers. (Maguire, Woollett, & Spiers, 2006)


- Birds with high memory demands have larger hippocampi
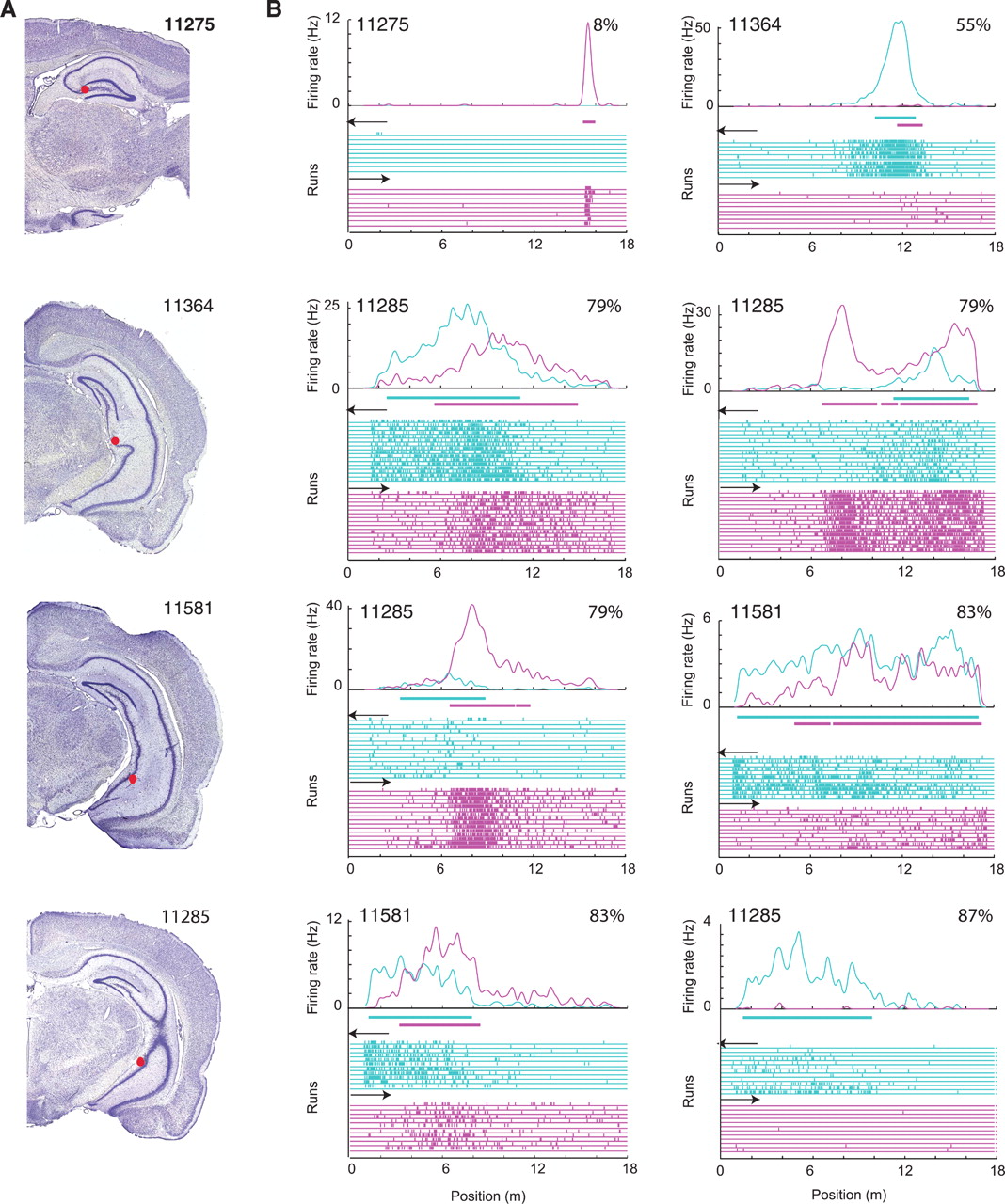
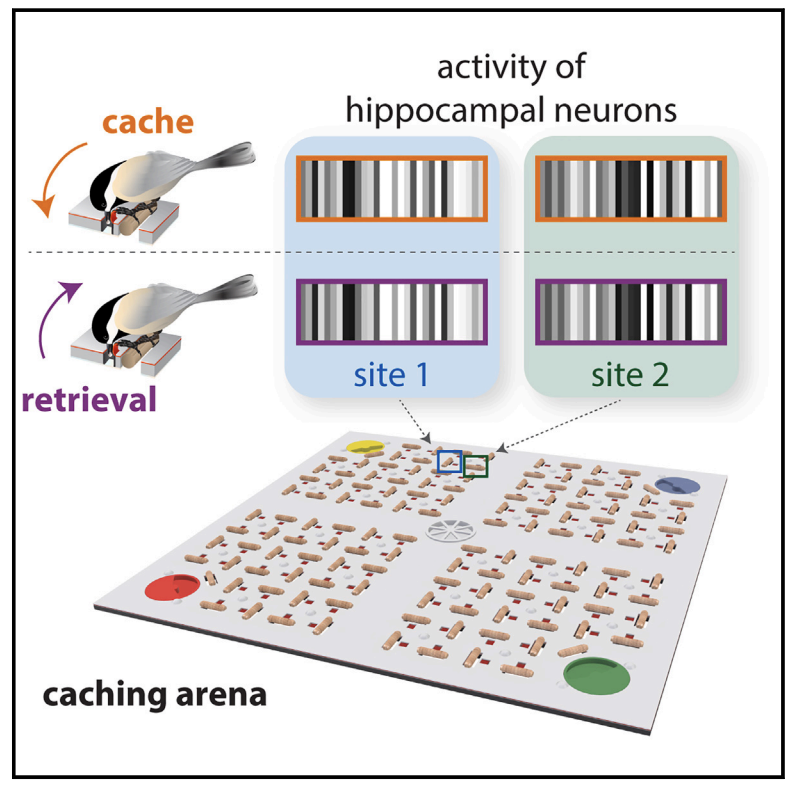
- Birds with high memory caching demands use sparse representations (“bar codes”) in hippocampus
- (Gilmore’s view) Humans have repurposed hippocampal system for other cognitive uses, e.g., semantic memory, language
Cerebral cortex
- Contains retinotopic maps (beyond V1) that are memory-related (Steel, Silson, Garcia, & Robertson, 2024)
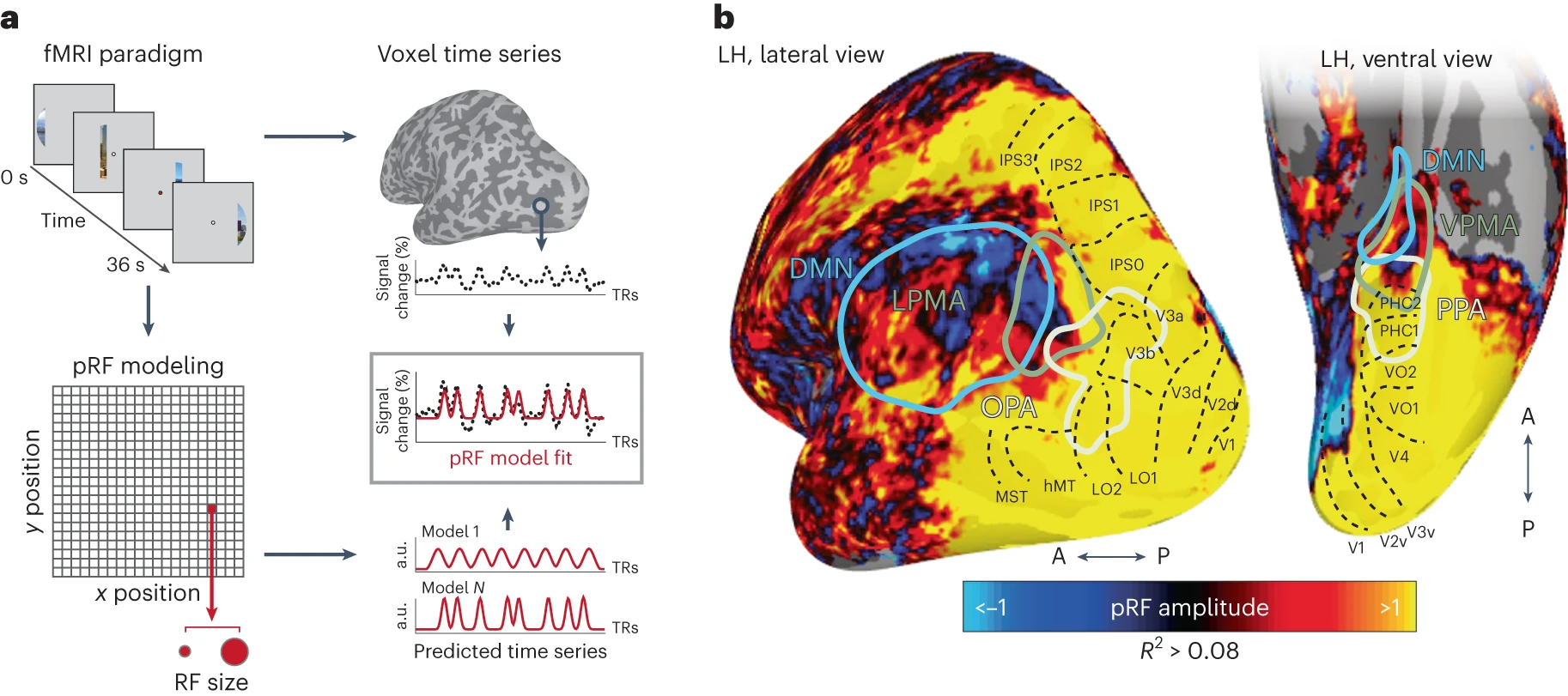
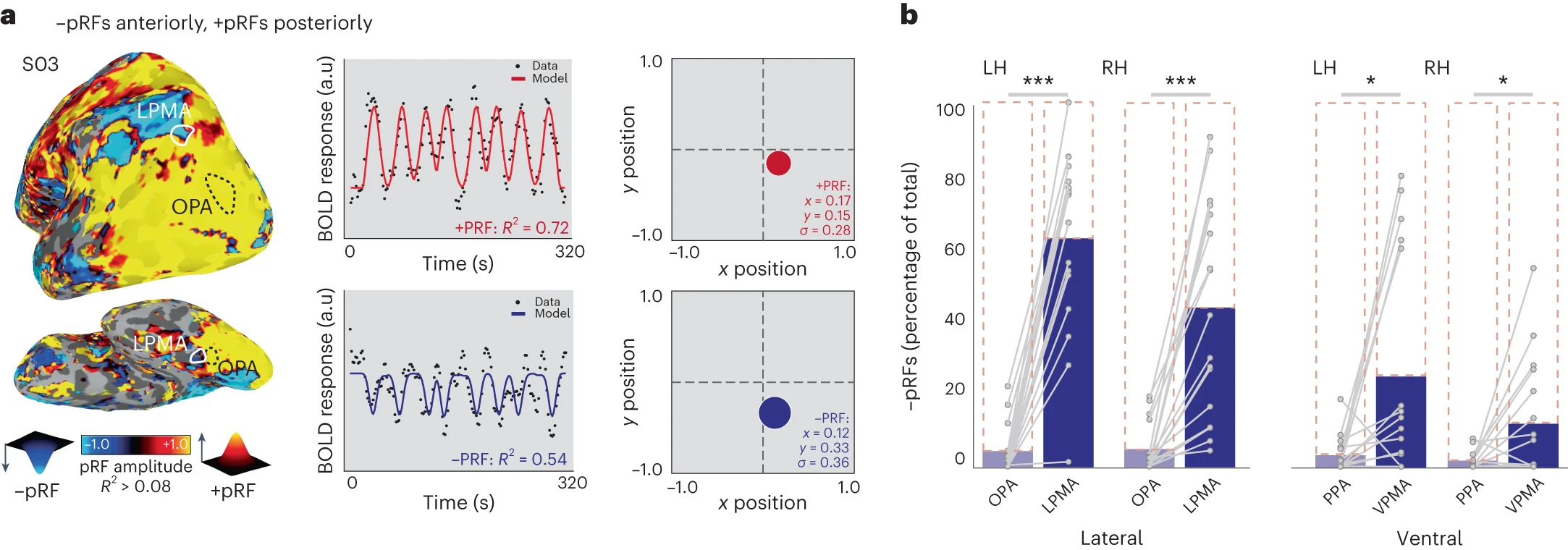
Together with recent reports showing retinotopic coding persisting as far as the ‘cortical apex’ [15,16,31], including the DMN [15,16], and the hippocampus [31,32], our findings challenge conventional views of brain organization, which generally assume that retinotopic coding is replaced by abstract amodal coding as information propagates through the visual hierarchy [7,8,9,10] toward memory structures [11,12,13,14] (Steel et al., 2024)
Cerebellum
- response timing, sequences, especially in classical conditioning paradigms Jirenhed, Rasmussen, Johansson, & Hesslow (2017)
Disorders of memory
Amnesia
- Acquired loss of memory
- ≠ normal forgetting
- Retrograde (‘backwards’ in time)
- Damage to information acquired pre-injury
- Temporally graded
- Anterograde (‘forward’ in time)
- Damage to information acquired/experienced post-injury
Patient HM (Henry G. Molaison)
- Intractable/untreatable epilepsy
- Bilateral resection of medial temporal lobe (1953)
- Epilepsy now treatable
- But, memory impaired
- Lived until 2008

HM’s amnesia
- Retrograde amnesia
- Can’t remember 10 yrs before operation
- Distant past better than more recent
- Severe, global anterograde amnesia
- Impaired learning of new facts, events, people
- But, skills (mirror learning) intact
“Every day is alone in itself, whatever enjoyment I’ve had, and whatever sorrow I’ve had…Right now, I’m wondering, have I done or said anything amiss? You see at this moment, everything looks clear to me, but what happened just before? That’s what worries me. It’s like waking from a dream. I just don’t remember.”
Other causes of amnesia
- Disease
- Alzheimer’s, herpes virus
- Korsakoff’s syndrome
- Result of severe alcoholism
- Impairs medial thalamus & mammillary bodies
Patient NA
- Fencing accident
- Damage to medial thalamus
- Anterograde + graded retrograde amnesia
- Are thalamus & medial temporal region connected?
Spared skills in amnesia
- Skill-learning
- Mirror-reading, writing
- Short-term memory
- “Cognitive” skills
- Priming
What does amnesia tell us?
- Long-term memory for facts, events, people
- ≠ Short-term memory
- ≠ Long-term memory for “skills”
- Separate memory systems in the brain?
Alzheimer’s Disease (AD)
- Chronic, neurodegenerative disease affecting ~5 M Americans
- Cognitive dysfunction (memory loss, language difficulties, planning, coordination)
- Psychiatric symptoms and behavioral disturbances
- Difficulties with daily living
- Burns & Iliffe (2009)
- Build up of amyloid \(\beta\) protein plaques in brain tissue
- APOE e4 gene variant increases risk 2-12x
Progression
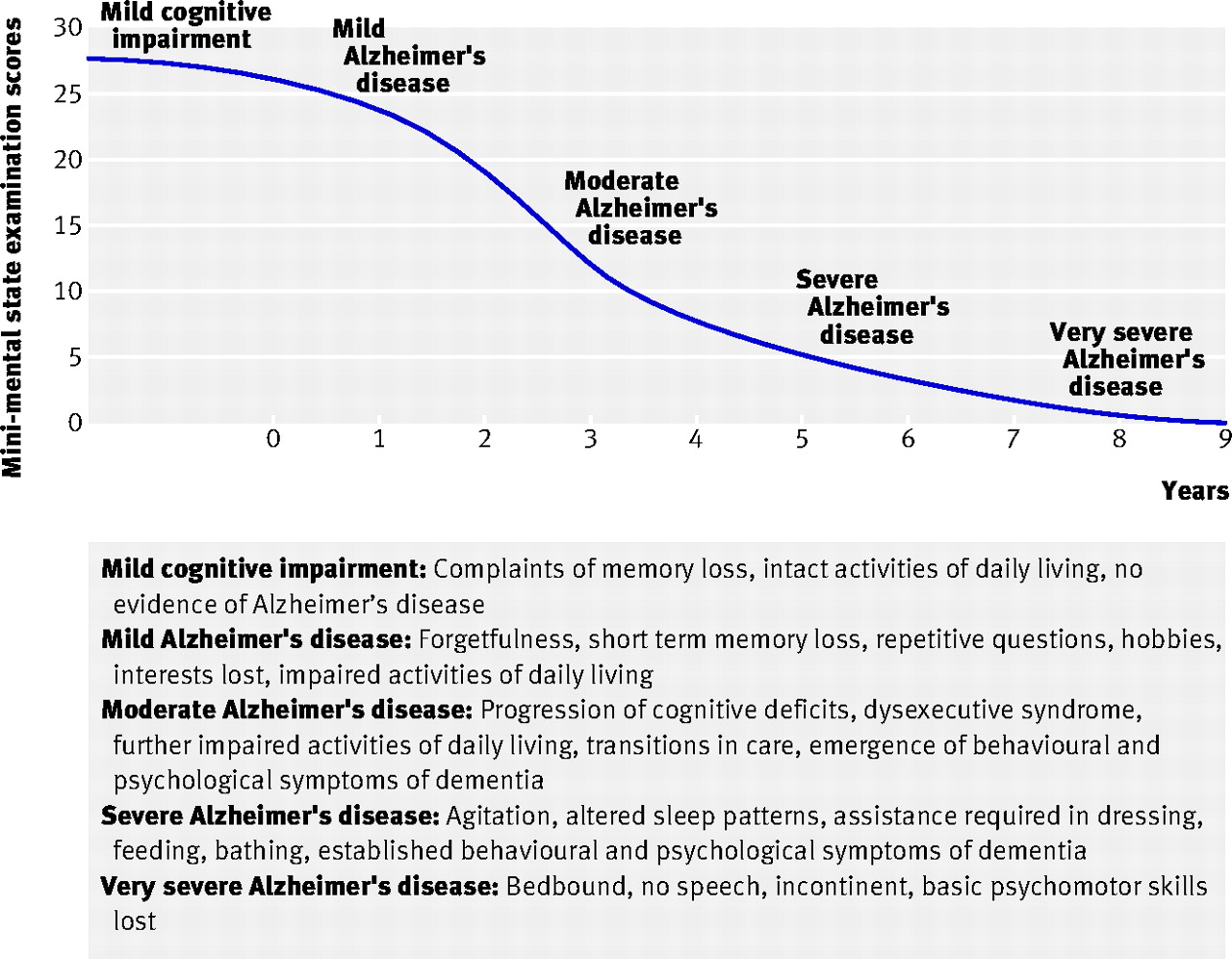
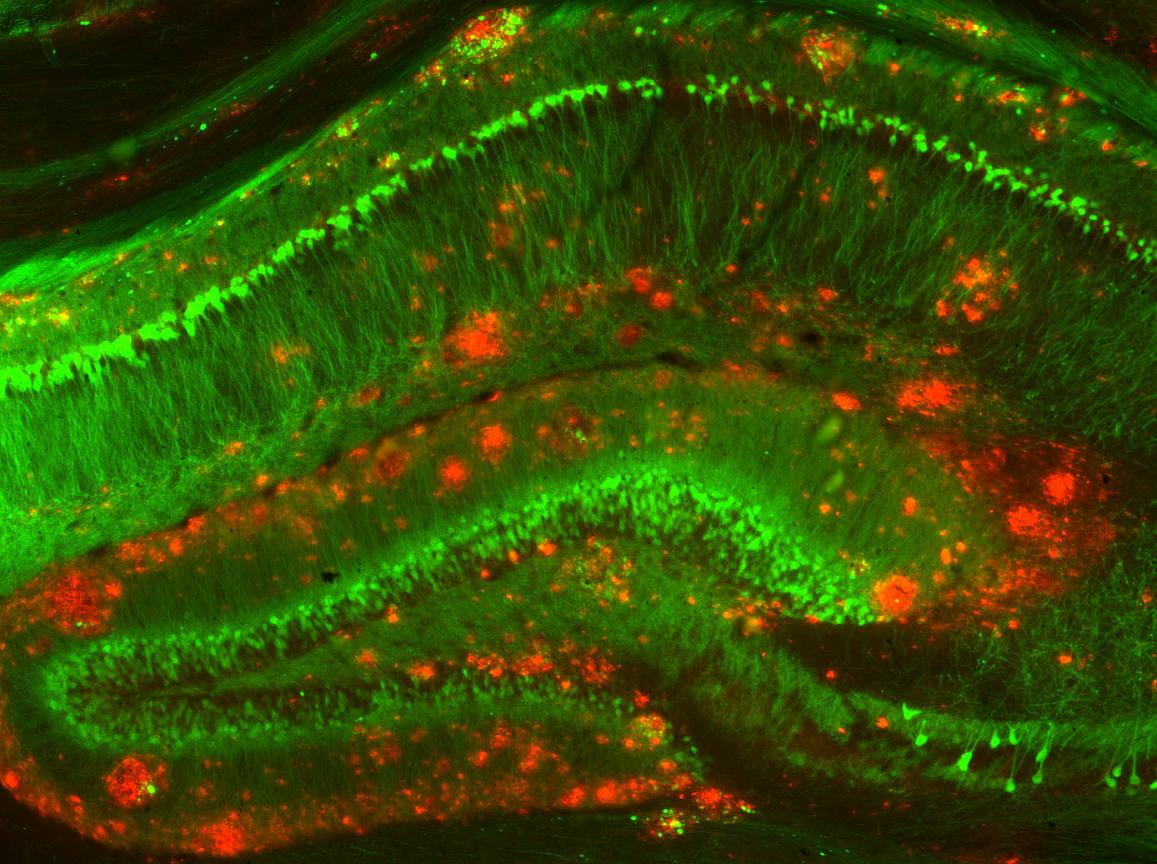
- Post-mortem exams show \(\beta\) amyloid plaques and neurofibrillary tangles
Treatments
- Acetylcholinesterase (AChE) inhbitors (e.g. Aricept): Slow ACh inactivation
- NMDA-R partial antagonists (e.g., Memantine): Slow LTP
- Drugs that remove amyloid \(\beta\) (e.g, lecanemab) don’t work especially well + have side effects
- AD the result of disordered immune response (Jevtic, Sengar, Salter, & McLaurin, 2017)?
- Promising prospective therapy using microglia to remove amyloid \(\beta\) (Hou et al., 2024)
- Link between genetic factor (APOE4/4 gene) and fatty build-up in microglia (Haney et al., 2024)
The other end of the spectrum…
What about working memory? (D’Esposito & Postle, 2015)
- LTM representations of target items + attention -> elevated activation
- Semantic items
- Sensorimotor items
- Capacity for attended items (in Focus of Attention or FoA) limited ~ 4
- Neural basis
- sustained activation in PFC
- subthreshold activation in areas where items are stored
- Individual differences in visual WM

Summary
- Multiple types of learning & memory
- Learning & memory distributed across the brain
- Specializations relate to location in the network? (input/output/in-between)
- Hippocampus + PFC critical areas binding together sensory/semantic info stored elsewhere
- Changes in synaptic #, strength, connectivity provide cellular basis