Neurochemistry
PSY 511.003
Neurotransmitters
What are they?
- Chemicals produced by neurons
- Released by neurons
- Bound by neurons and other cells
- Send messages (have physiological effect on target cells)
- Inactivated after release
Things to know
- Neurotransmitter
- Where released from/to
- What receptor(s) bind it
Amino acids
| Family | Neurotansmitter |
|---|---|
| Amino acids | Glutamate |
| \(\gamma\) aminobutyric acid (GABA) | |
| Glycine | |
| Aspartate |
Glutamate
- Widespread in CNS (~ 1/2 all synapses)
- Primary excitatory NT in CNS
- Role in learning (via NMDA receptor)
- Receptors on neurons and glia (astrocytes and oligodendrocytes)
- Linked to umami (savory) taste sensation (think monosodium glutamate or MSG)
- Dysregulation in schizophrenia (McCutcheon, Krystal, & Howes, 2020), mood disorders (Małgorzata, Paweł, Iwona, Brzostek, & Andrzej, 2020)
| Type | Receptor | Esp Permeable to |
|---|---|---|
| Ionotropic | AMPA | \(Na^+\), \(K^+\) |
| Kainate | ||
| NMDA | \(Ca^{++}\) | |
| Metabotropic | mGlu |
\(\gamma\) aminobutyric acid (GABA)
- Primary inhibitory NT in CNS
- Excitatory in developing CNS, [\(Cl^-\)] in >> [\(Cl^-\)] out
- Binding sites for benzodiazepines (BZD; e.g., Valium), barbiturates, ethanol, etc.
- BZD affect subset of GABA-A receptors
- Increase total Cl- influx
| Type | Receptor | Esp Permeable to |
|---|---|---|
| Ionotropic | GABA-A | \(Cl^-\) |
| Metabotropic | GABA-B | \(K^+\) |


Other amino acid NTs
- Aspartate
- Like Glu, stimulates NMDA receptor
- Glycine
- Spinal cord interneurons
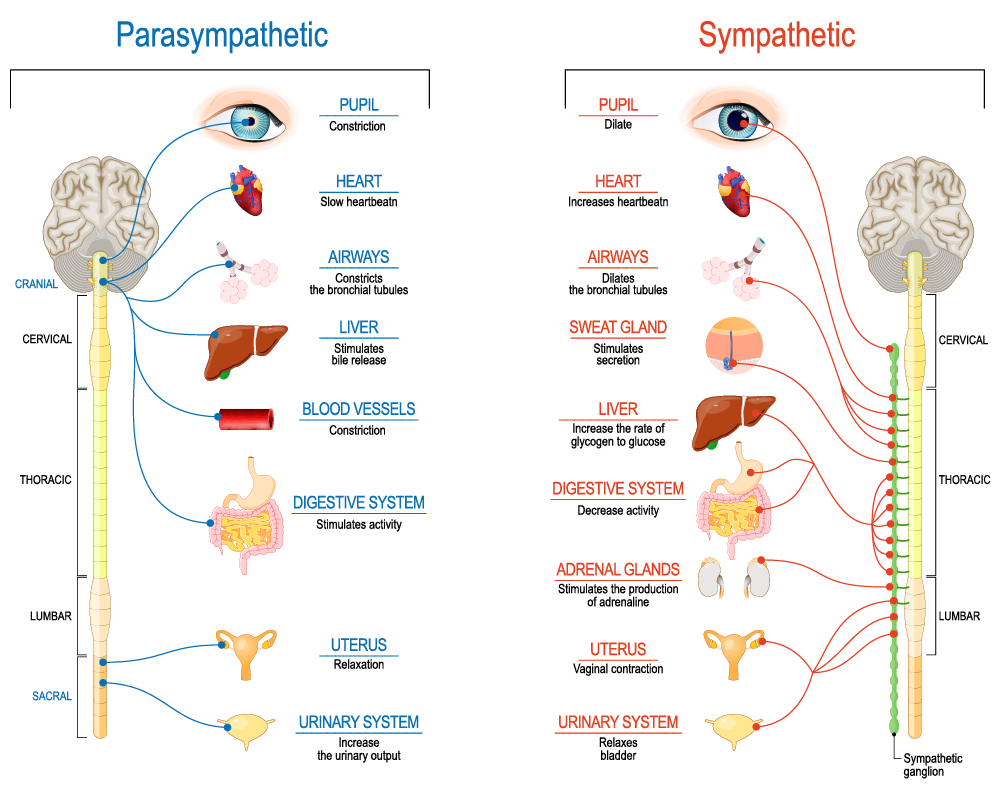
Acetylcholine (ACh)
- Primary excitatory NT of CNS output
- Somatic nervous system (motor neuron -> neuromuscular junction)
- Autonomic nervous system (ANS)
- Sympathetic branch: preganglionic neuron
- Parasympathetic branch: pre/postganglionic
| Type | Receptor | Esp Permeable to | Blocked by |
|---|---|---|---|
| Ionotropic | Nicotinic (nAChR) | \(Na^+\), \(K^+\) | e.g., Curare |
| Metabotropic | Muscarinic (mAChR) | \(K^+\) | e.g., Atropine |
Curare


Atropine
- aka, nightshade or belladonna
- inhibits (acts as an antagonist for) muscarinic ACh receptor


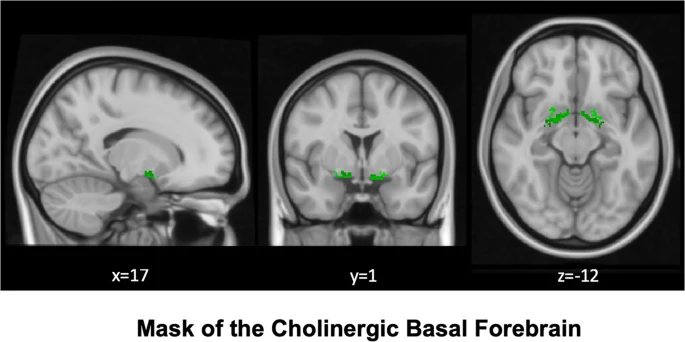
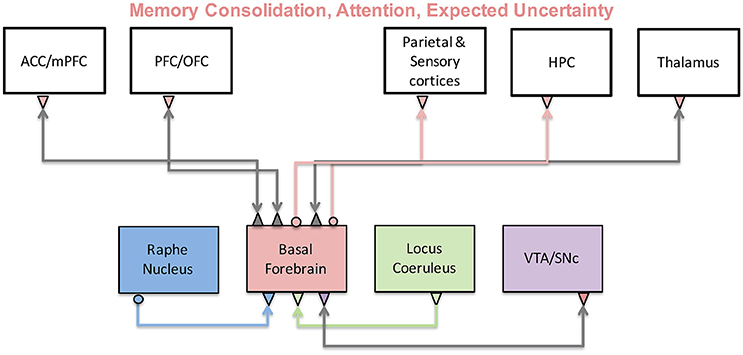
Basal forebrain

Monoamine NTs
| Family | Neurotransmitter | Comment |
|---|---|---|
| Monoamines | Dopamine (DA) | Catecholamine |
| Norepinephrine (NE)/Noradrenaline (NAd) | Catecholamine | |
| Epinephrine (Epi)/Adrenaline (Ad) | Catecholamine | |
| Serotonin (5-HT) | Indolamine | |
| Melatonin | Indolamine | |
| Histamine |
- Synthesis pathway: DA -> NE/NAd -> Epi/Ad
Information processing
- Point-to-point
- One sender, small number of recipients
- Glu, GABA
- Broadcast
- One sender, widespread recipients
- DA, NE, 5-HT, melatonin, histamine
- Need to know
- NT, where projecting, type of receptor to predict function
Dopamine
- Released by
- Substantia nigra -> striatum, meso-striatal projection
- Ventral tegmental area (VTA) -> nucleus accumbens, ventral striatum, hippocampus, amygdala, cortex; meso-limbo-cortical projection

Clinical relevance
- Parkinson’s Disease (mesostriatal)
- DA agonists treat (agonists facilitate/increase transmission)
- ADHD (mesolimbocortical)
- Schizophrenia (mesolimbocortical)
- DA antagonists treat
- Addiction (mesolimbocortical)
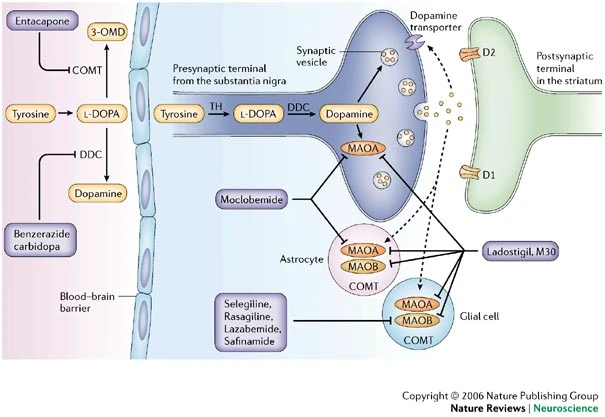
Inactivated via
- Chemical breakdown (e.g., via monoamine oxidase (MAO)), http://www.scholarpedia.org/article/Dopamine_anatomy#Dopamine_receptors
- Dopamine transporter (DAT)
- Psychostimulants (e.g., cocaine, methylphenidate) act upon. (“Dopamine transporter,” n.d.)
- DAT also transports norepinephrine (NE)

| Type | Receptor | Comments |
|---|---|---|
| Metabotropic | D1-like (D1 and D5) | more prevalent |
| D2-like (D2, D3, D4) | target of many antipsychotics |
Norepinephrine
- Released by
- locus coeruleus in pons/caudal tegmentum
- postganglionic sympathetic neurons onto target tissues


- Role in arousal, mood, eating, sexual behavior
Clinical relevance
- ADHD, Alzheimer’s Disease, Parkinson’s Disease, depression
Inactivated by
- Norepinephrine transporter (NET), aka noradrenaline transporter (NAT)
- Contributes to DA uptake, too.
- Also monoamine oxidase inhibitors (MAOIs)
- inactivate monoamines in neurons, astrocytes
- MAOIs increase NE, DA
- Treatment for depression
| Type | Receptor | Comments |
|---|---|---|
| Metabotropic | \(\alpha\) (1,2) | antagonists treat anxiety, panic |
| \(\beta\) (1,2,3) | ‘beta blockers’ in cardiac disease |
Adrenaline/Epinephrine
- Synthesized from norepinephrine
- Both NT and hormone
- As NT: Released in small amounts by medulla oblongata
- As hormone: Released by adrenal medulla
- Binds to (\(\alpha_{1,2}\), \(\beta_{1,2,3}\)) receptors in blood vessels, cardiac muscle, lungs, eye muscles controlling pupil dilation, liver, pancreas, etc.
- Release enhanced by cortisol from adrenal cortex
- Unusual in NOT being part of negative feedback system controlling its own release
Serotonin (5-hydroxytryptamine or 5-HT)
- Released by raphe nuclei in brainstem

- Role in mood, sleep, eating, pain, nausea, cognition, memory
- Modulates release of other NTs
- Most (90%; (De Ponti, 2004)) of body’s 5-HT regulates digestion
- Separate cortical, subcortical 5-HT projection pathways?
- Seven receptor families (5-HT 1-7) with 14 types
- All but one metabotropic
Clinical relevance
- Ecstasy (MDMA) disturbs serotonin
- So does LSD
- Fluoxetine (Prozac)
- Selective Serotonin Reuptake Inhibitor (SSRI)
- Treats depression, panic, eating disorders, others
- 5-HT3 receptor antagonists are anti-mimetics used in treating nausea

Public Domain, Link
- Different psychological roles (passive vs. active coping) associated with different 5-HT receptor subtypes? (Carhart-Harris & Nutt, 2017)
Comparisons among neuromodulators
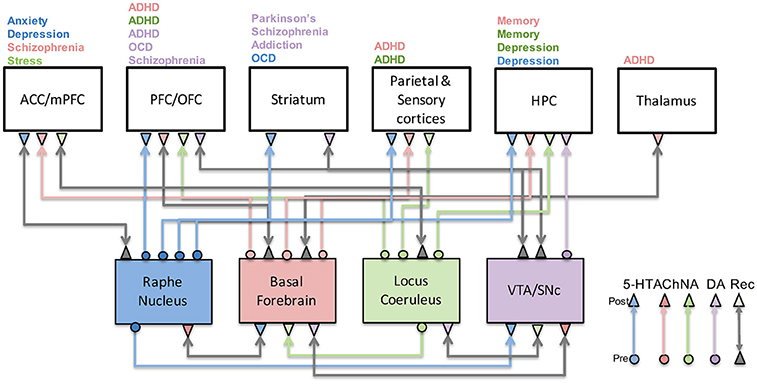
- Limited evidence for specific functions by neuromodulator
- Same neuromodulators -> different effects on different target areas
- Most neuromodulators relate to attention and novelty detection
- Neuromodulators interact with one another
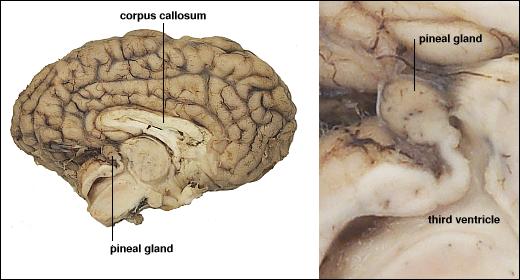
Melatonin
- Released by pineal gland (pine cone-like appearance)
Histamine
- Released by hypothalamus, projects to whole brain.
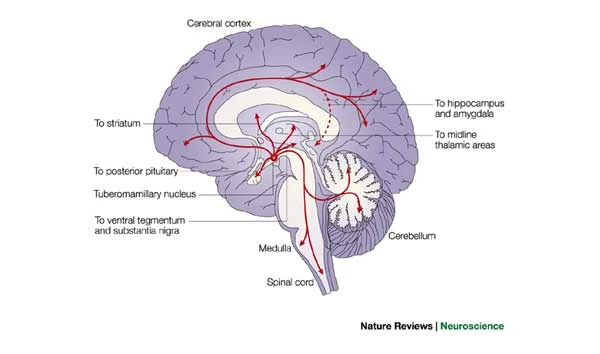
- \(H_1\)-\(H_4\) Metabotropic receptors, one ionotropic type in thalamus/hypothalamus
- Role in arousal/sleep regulation
- In body, part of immune/inflammatory response
Targets of psychotropic drugs
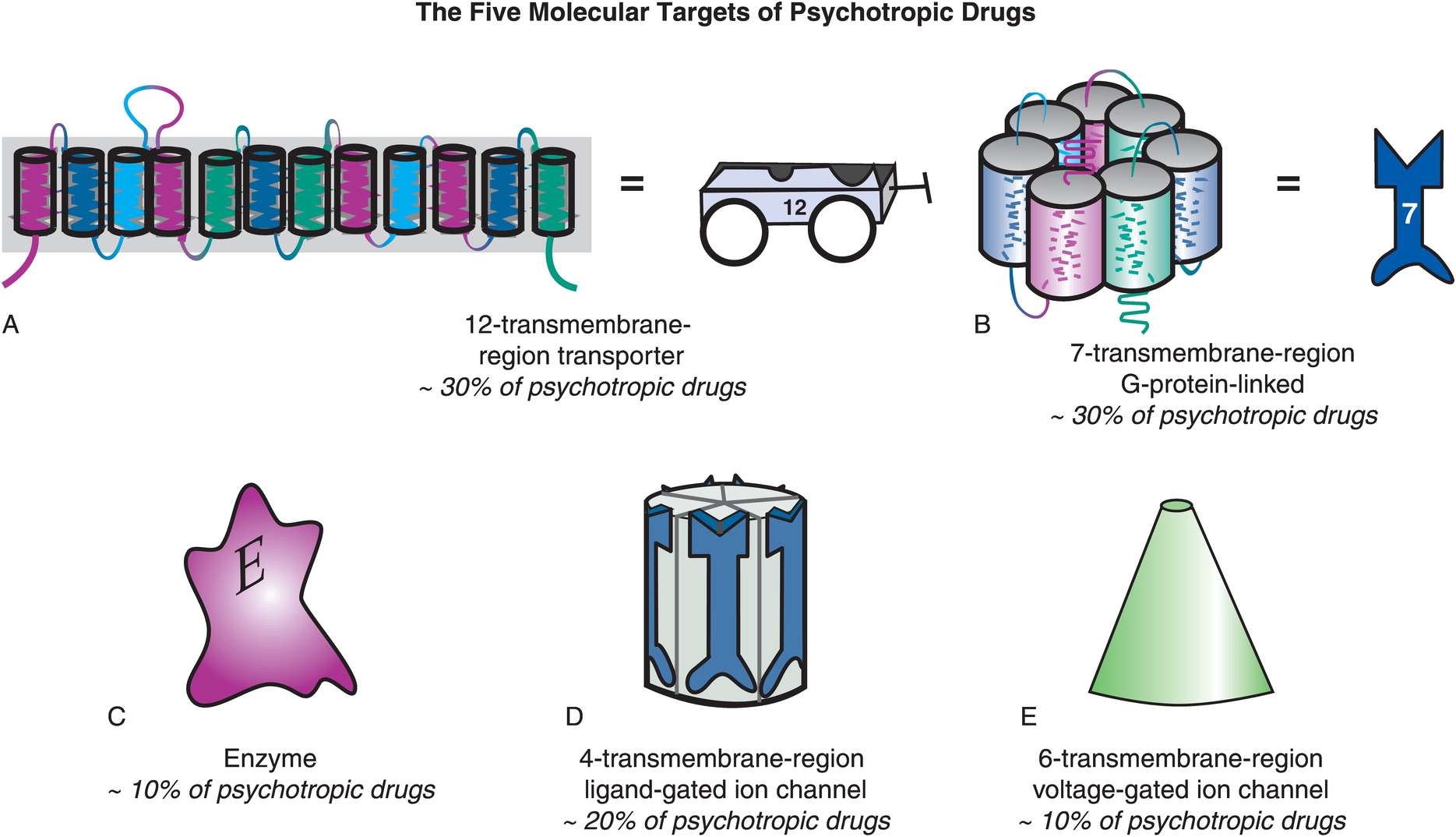
- Transporters
- G-protein-linked (metabotropic receptors)
- Enzymes
- Ligand-gated channels
- Voltage-gated (ionotropic receptors)
Other NTs
- Gases
- Nitric Oxide (NO), carbon monoxide (CO)
- Neuropeptides
- Substance P and endorphins (endogenous morphine-like compounds) have role in pain
- Orexin/hypocretin, project from lateral hypothalamus across brain, regulates appetite, arousal
- Cholecystokinin (CCK) stimulates digestion
- Purines
- Adenosine (inhibited by caffeine)
- Others
- Anandamide (activates endogenous cannabinoid receptors)
Hormonal communication
- Chemicals secreted into blood
- Act on specific target tissues via receptors
- Produce specific effects
Examples of substances that are both hormones and NTs
- Melatonin
- Epinephrine/adrenaline
- Oxytocin
- Arginine Vasopressin (AVP) or Anti-Diuretic Hormone (ADH)
Behaviors under hormonal influence
Ingestive (eating/ drinking)
- Fluid levels
- Na, K, Ca levels
- Digestion
- Blood glucose levels
Responses to threat/challenge
- Metabolism
- Heart rate, blood pressure
- Digestion
- Arousal
Common factors
- Biological imperatives
- Proscribed in space and time
- Foraging/hunting
- Find targets distributed in space, evaluate, act upon
- Often involve others
Principles of hormonal action
- Gradual action
- Change intensity or probability of behavior
- Behavior influences/influenced by hormones
- +/- Feedback
- Multiple effects on different tissues
- Produced in small amounts; released in bursts
- Levels vary daily, seasonally
- or are triggered by specific external/internal events
- Effect cellular metabolism
- Influence only cells with receptors
- Point to point vs.“broadcast”
- Wider broadcast than neuromodulators
Similarities between neural and hormonal communication
- Chemical messengers stored for later release
- Release follows stimulation
- Action depends on specific receptors
- 2nd messenger systems common
Hormonal release sites
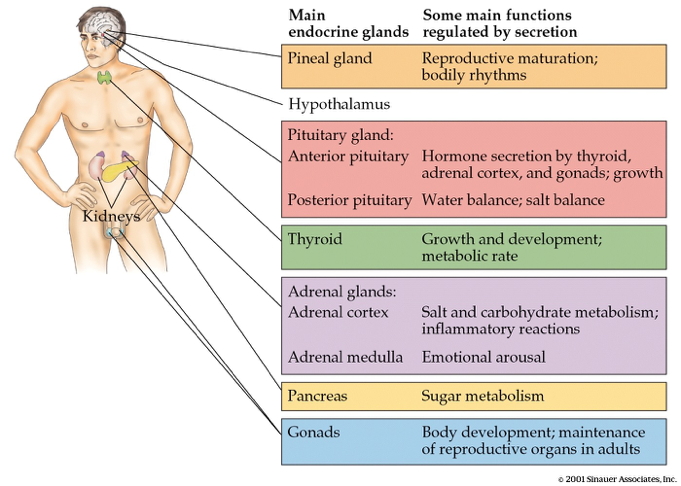
- CNS
- Hypothalamus
- Pituitary
- Anterior
- Posterior
- Pineal gland
- Rest of body
- Thyroid
- Adrenal (ad=adjacent, renal=kidney) gland
- Adrenal cortex
- Adrenal medulla
- Gonads (testes/ovaries)
Two release systems from hypothalamus
Direct release
- Hypothalamus (paraventricular, supraoptic nucleus) to
- Posterior pituitary
- Oxytocin
- Arginine Vasopressin (AVP, vasopressin)

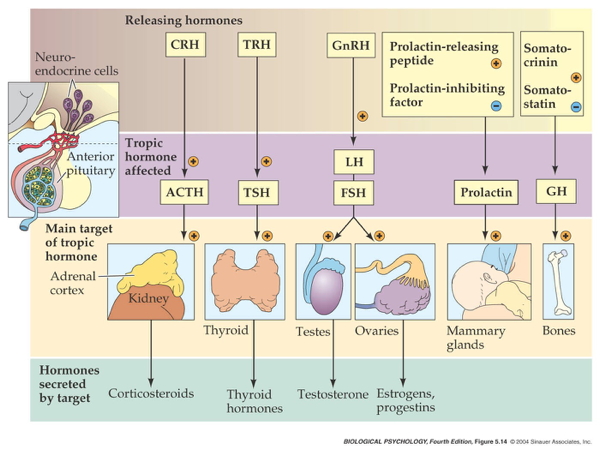
Indirect release
- Hypothalamus -> releasing hormones
- Anterior pituitary -> tropic hormones
- End organs
Case studies
Responses to threat or challenge
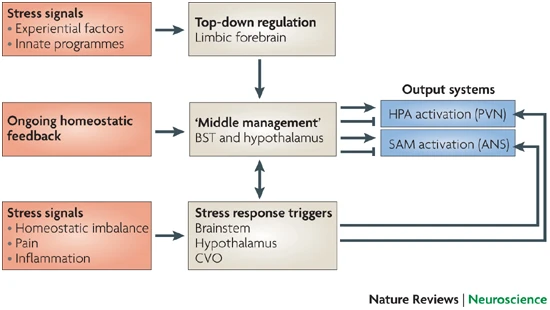
- Neural response
- Sympathetic Adrenal Medulla (SAM) response
- Sympathetic NS activation of adrenal medulla, other organs
- Releases NE and Epi into bloodstream
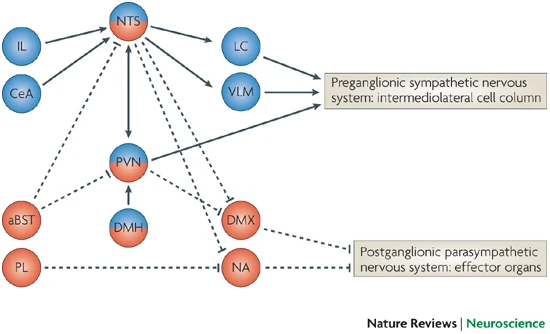
- Endocrine response
- Hypothalamic Pituitary Adrenal (HPA) axis
- Adrenal hormones released

- Hypothalamus
- Corticotropin Releasing Hormone (CRH) or Corticotropin Releasing Factor (CRF)
- Paraventricular nucleus (PVN)
- Anterior pituitary
- Adrenocorticotropic hormone (ACTH)
- Adrenal cortex
- Glucocorticoids (e.g., cortisol)
- Mineralocorticoids (e.g. aldosterone)
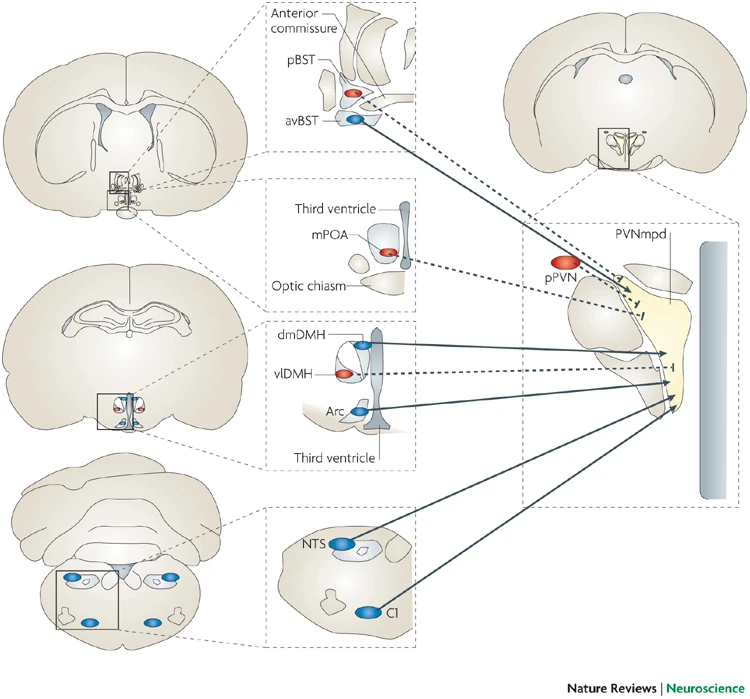
- CRF receptors found throughout the brain

Adrenal hormones
- Steroids
- Derived from cholesterol
- Cortisol (CORT)
- increases blood glucose, aids in fat, protein, & carbohydrate metabolism
- suppressess immune response, e.g., anti-inflammatory
- in presence of Epi/Ad, role in memory formation
- Receptors found in cytosol of most cells; some on cell membranes
- Regulates gene transcription
- circadian rhythmicity: high in am, low in pm

- Aldosterone
- Regulates Na (and water)
Reproductive behavior – the milk letdown reflex
- Supraoptic nucleus & Paraventricular nucleus (PVN) of hypothalamus release oxytocin
- Into bloodstream via posterior pituitary (endocrine)
- Onto neurons in nucleus accumbens (neurocrine), amygdala, brainstem
- Oxytocin stimulates milk ducts to secrete
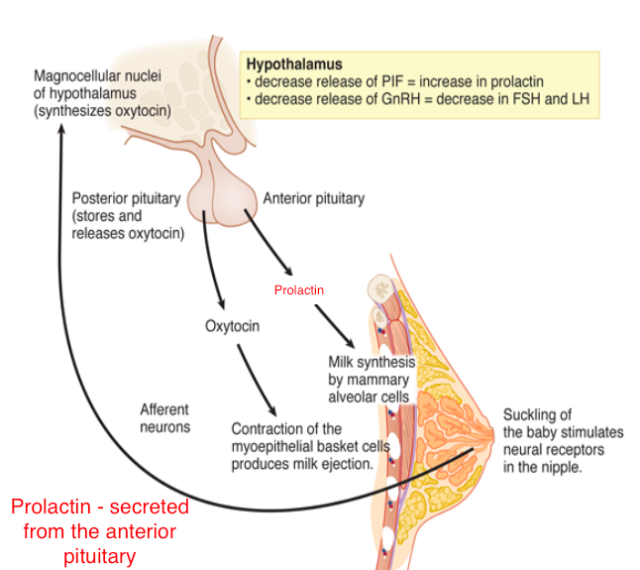
Oxytocin’s role…
- Sexual arousal
- Released during orgasm, causing rhythmic muscle contractions
- Stimulates uterine, vaginal contraction during labor
- But mouse OXY knock-out model still engages in reproductive behavior and gives birth without incident.
- Oxytocin-producing cells in ovarian corpus luteum, testicles, retina, adrenal medulla, pancreas
- Links to social interaction, bonding (Weisman & Feldman, 2013)
- Alters face processing in autism (Domes et al., 2013)
- May inhibit fear/anxiety-related behaviors by gating amygdala (Viviani et al., 2011)
.png)
Circadian rhythms
Melatonin
- Diurnal rhythm
- Night time peak, early morning low
- Secretion suppressed by short wavelength or “blue” light (< 460-480 nm)
- Rhythm irregular until ~3 mos post-natal (Ardura, Gutierrez, Andres, & Agapito, 2003)
- Peak weakens, broadens with age
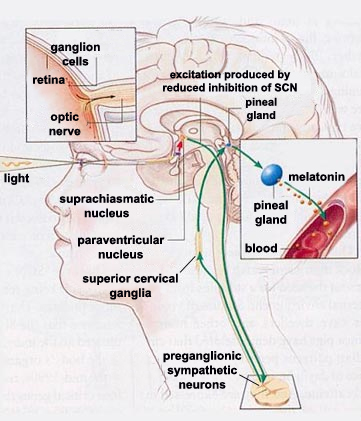
- Pathway
- Suprachiasmatic nucleus (SCN) of the hypothalamus
- Paraventricular nucleus of the hypothalamus
- Spinal cord
- Superior cervical ganglion
- Pineal gland
Thinking about neurochemical influences
- Measure hormones in blood, saliva, urine; can’t effectively measure NTs
- Multivariate, nonlinear, mutually interacting
- Varied time scales
- Phasic (e.g., cortisol in response to challenge)
- Periodic (e.g., melatonin; diurnal cortisol)
- Peripheral effects + neural feedback
- State variables and behavior
- Are your participants sleepy, hungry, horny, distressed…
- Endogenous & exogenous influences
- Systems interact; need better, broader, and denser measurement
References
Ardura, J., Gutierrez, R., Andres, J., & Agapito, T. (2003). Emergence and evolution of the circadian rhythm of melatonin in children. Horm. Res., 59(2), 66–72. https://doi.org/68571
Avery, M. C., & Krichmar, J. L. (2017). Neuromodulatory systems and their interactions: A review of models, theories, and experiments. Frontiers in Neural Circuits, 11, 108. https://doi.org/10.3389/fncir.2017.00108
Avram, M., Grothe, M. J., Meinhold, L., Leucht, C., Leucht, S., Borgwardt, S., … Sorg, C. (2021). Lower cholinergic basal forebrain volumes link with cognitive difficulties in schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. https://doi.org/10.1038/s41386-021-01070-x
Carhart-Harris, R. L., & Nutt, D. J. (2017). Serotonin and brain function: A tale of two receptors. Journal of Psychopharmacology, 31(9), 1091–1120. https://doi.org/10.1177/0269881117725915
De Ponti, F. (2004). Pharmacology of serotonin: What a clinician should know. Gut, 53(10), 1520–1535. https://doi.org/10.1136/gut.2003.035568
Deussing, J. M., & Chen, A. (2018). The Corticotropin-Releasing factor family: Physiology of the stress response. Physiological Reviews, 98(4), 2225–2286. https://doi.org/10.1152/physrev.00042.2017
Domes, G., Heinrichs, M., Kumbier, E., Grossmann, A., Hauenstein, K., & Herpertz, S. C. (2013). Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry, 74(3), 164–171. https://doi.org/http://dx.doi.org/10.1016/j.biopsych.2013.02.007
Haas, H., & Panula, P. (2003). The role of histamine and the tuberomamillary nucleus in the nervous system. Nature Reviews. Neuroscience, 4(2), 121–130. https://doi.org/10.1038/nrn1034
Małgorzata, P., Paweł, K., Iwona, M. L., Brzostek, T., & Andrzej, P. (2020). Glutamatergic dysregulation in mood disorders: Opportunities for the discovery of novel drug targets. Expert Opinion on Therapeutic Targets, 24(12), 1187–1209. https://doi.org/10.1080/14728222.2020.1836160
McCutcheon, R. A., Krystal, J. H., & Howes, O. D. (2020). Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry: Official Journal of the World Psychiatric Association, 19(1), 15–33. https://doi.org/10.1002/wps.20693
Mesulam, M.-M. (2013). Cholinergic circuitry of the human nucleus basalis and its fate in alzheimer’s disease. The Journal of Comparative Neurology, 521(18), 4124–4144. https://doi.org/10.1002/cne.23415
Ren, J., Friedmann, D., Xiong, J., Liu, C. D., Ferguson, B. R., Weerakkody, T., … Luo, L. (2018). Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell. https://doi.org/10.1016/j.cell.2018.07.043
Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. https://doi.org/10.1038/nrn2647
Viviani, D., Charlet, A., Burg, E. van den, Robinet, C., Hurni, N., Abatis, M., … Stoop, R. (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science, 333(6038), 104–107. https://doi.org/10.1126/science.1201043
Weisman, O., & Feldman, R. (2013). Oxytocin effects on the human brain: Findings, questions, and future directions. Biological Psychiatry, 74(3), 158–159. https://doi.org/http://dx.doi.org/10.1016/j.biopsych.2013.05.026
Youdim, M. B. H., Edmondson, D., & Tipton, K. F. (2006). The therapeutic potential of monoamine oxidase inhibitors. Nature Reviews. Neuroscience, 7(4), 295–309. https://doi.org/10.1038/nrn1883
